*Webmaster’s note: I started with Greg Reese’s Report, and researched out the points in his video.
The content on this page reviews 2 seemingly separate topics. GMO – Genetic Editing of Food & mRNA/vaccines in our food and water.
American Farmers To Begin Injecting Livestock With mRNA Shots This Month. REESE REPORT
There are no laws requiring informed consent for vaccine food.
- Medicago
- Philip Morris: Medicago
- American Farmers To Begin Injecting Livestock This Month
- FDA okays genome-edited beef cattle trait
- Dr Naomi Wolf & Lawyer Thomas Renz
- Emerging trends of edible vaccine therapy 2022
- UK Precision Breeding Act 2023
- Precision Breeding Bill. Nothing to see here
- A sleight mistake: Precision Breeding Bill
- “Green King” Gives Royal Assent to Precision Breeding Bill
- Lab-Grown Meat Is Made of Cancer Cells
- Darkfield Blood Analysis On Grocery Meat Products
- Keeping our Eye on Greenlight Biosciences, a Bill & Melinda Gates Foundation Partner
- Orchestration of a Pandemic Famine
- Robert Malone mRNA vaccines for livestock, pets & wildlife
- Feeding Spray-Dried Porcine Plasma to Pigs Improves the Protection Afforded by the African Swine Fever Virus
February 3, 2023 Cessation of Operations at Medicago
[Medicago mentioned at 45 seconds into Greg Reese Video]
The Mitsubishi Chemical Group (“the Group”) has announced by press release published on February 3rd,2023 (9am local time in Japan) that it has decided to cease all its operations at Medicago Inc., Medicago R&D Inc. and Medicago USA Inc.
As the sole shareholder in Medicago, the Group has determined not to make further investments in Medicago and to proceed with an orderly wind-up of its business and operations in Canada and in the United States.
Medicago wishes to thank all its employees for their commitment, their passion, and their dedication.
The Medicago team has pushed scientific boundaries and we know that they will continue to make incredible contributions to innovation and biopharmaceutical’s sector. We also wish to thank all Canadians who supported our journey, as well as the governments of Canada and Quebec, our valued business partners, suppliers, vendors, and communities.
| Greg Reese Apr 6 Yes I saw that [Medicago closed] in my research. Medicago shut down due to “significant changes to the COVID-19 vaccine landscape” such as the “market environment”. In other words, the public doesn’t want to hear about vaccines. Phillip Morris was the parent company and they now own the technology. |
https://medicago.com/en/press-release/cessation-of-operations-at-medicago/
July 22, 2021 – Medicago employees have been working hard for 16 months to develop a vaccine candidate against the SARS-CoV-2 virus. With funding of $ 173 million from the federal government, the company hoped to reap the rewards of its efforts this summer by obtaining approval from Health Canada. https://www.archyde.com/due-to-lack-of-candidates-medicago-lags-behind-in-its-clinical-trial-coronavirus/
The clinical and technological advances we [Medicago] have made are, in large part, due to the close and lasting partnerships that Medicago’s team has fostered over the years.
We are a trusted partner of health authorities around the globe. We have partnered with US governmental agencies, DARPA and BARDA. The Canadian government has provided significant funding for the development of our COVID-19 vaccine candidate and the construction of our new large-scale manufacturing facility in Quebec, Canada.
We have worked with governmental agencies in Europe and Latin America to conduct clinical trials.
~ ~ ~ ~ ~ ~ ~ ~
This information is intended for Canadian residents.
COVIFENZ®
COVID-19 Vaccine (plant-based virus-like particles [VLP], recombinant, adjuvanted)
Click here to see the COVIFENZ product monograph. This product monograph is intended for use by healthcare professionals in Canada. [See document here]
Click here to see the COVIFENZ patient medication information.
Canadian regulations limit the information we are permitted to give on prescription drugs on digital platforms.
[*noted: This is administered intramuscularly into the deltoid muscle. Therefore not eaten ~Webmaster’s Observation]
Vaccine Choice Canada
Dr. Chris Shaw Evaluates Novavax: nuvaxovid and Medicago: covifenz

Please consult a healthcare professional for more detailed information and to discuss the appropriateness of a particular vaccine.
For medical information or to report a side effect or product complaint, please call Medicago at 1-800-622-6067 or email medinfo.can@medicago.com and to Health Canada as well through the Canada Vigilance Program at 1-866-234-2345.
[*noted: This is administered intramuscularly into the deltoid muscle. Therefore not eaten ~Webmaster’s Observation]
More info: https://gregreese.substack.com/p/american-farmers-to-begin-injecting
More info: https://medicago.com/en/our-company/our-pandemic-response/
Philip Morris International and Medicago’s COVID-19 Vaccine
Business of Tobacco [March 17, 2022]
Medicago is a Canadian biotechnology company, 25% owned by Philip Morris International (PMI). In October 2020, the Canadian government invested millions in Medicago for the development of a COVID-19 vaccine. As of March 2022, Medicago’s tobacco plant-derived vaccine has received marketing approval from Health Canada and clinical trials are continuing in several other countries.
PMI has praised the “public private partnership,” between Medicago and the Canadian government, raising concerns that PMI may leverage Medicago for corporate marketing, to gain access to policymakers and to complicate WHO FCTC implementation.
In a regular press briefing on March 16, 2022, World Health Organization confirmed that it was not progressing an Emergency Use Listing application from Medicago because of its links to PMI. This means that Medicago’s vaccine is not eligible for inclusion in the COVAX vaccine supply scheme.
Learn more, plus how governments can counter the industry’s tactics to get close to policymakers.
Read the brief:
Quote from the brief: “The Canadian government’s investment in Medicago is through another department, Science & Economic Development (ISED), which committed up to CAD $173 million (US $131 million) for vaccine research and development and to construct a manufacturing facility in Quebec City. This was dubbed one of the largest federal government investments in vaccine development. This has the effect of positioning PMI, via Medicago, as a partner of the Canadian government, which is viewed as a global leader in tobacco control.“
WHO Framework Convention on Tobacco Control
American Farmers To Begin Injecting Livestock With mRNA Shots This Month [Mentioned at 1:38 in Greg Reese Video]
mRNA Vaccines for Livestock?
Are mRNA vaccines being developed for livestock? You bet they are! So what does this mean? As usual, it depends who you ask. Find out the bad, the worse and the putrid of third generation vaccines and the future of food in this week’s edition of Questions For Corbett.
Show notes: https://www.corbettreport.com/qfc-livestock/
*Webmaster’s note: I was able to find mentioned items. This video sums up what I found very well.
You can follow the links he provides.
NSW Australian farmers say they are not being mandated to give mRNA vaccines to their livestock… YET. Corbett mentions this story.
mRNA Vaccines for Livestock? – Questions For Corbett #097 03/02/2023
mRna, Rna particle and DNA vaccines have been researched for more then 12 years. *no indication of approval as of Jan 2023. (this video)
If the Gateses and the Faucis and the representatives of the international medical establishment get their way, life will not return to normal until the entire planet is vaccinated against SARS-CoV-2. What many do not yet understand, however, is that the vaccines that are being developed for SARS-Cov-2 are unlike any vaccines that have ever been used on the human population before. And, as radically different as these vaccines appear, they represent only the very beginning of a complete transformation of vaccine technology that is currently taking place in research labs across the planet. This is a study of The Future of Vaccines.
The Future of Vaccines: The Corbett Report (mentioned above Dec. 23/2020)
Transcript: https://www.corbettreport.com/futurevaccines/
Moderna Whitepaper:
National Cattlemen’s Beef Association (NCBA) Statement Correcting Internet Falsehoods About mRNA Vaccines in Cattle
April 5, 2023 [Mentioned at 1:38 in Greg Reese Video]
WASHINGTON (April 5, 2023) – Today, the National Cattlemen’s Beef Association (NCBA) released a statement in regard to false information circulating on social media about the use of mRNA vaccines in cattle:
“There are no current mRNA vaccines licensed for use in beef cattle in the United States. Cattle farmers and ranchers do vaccinate cattle to treat and prevent many diseases, but presently none of these vaccines include mRNA technology.”
Dr. Mike Adams: https://www.brighteon.com/fdfc0a04-0872-4c75-8f08-38d4169d5574
~~~~~~~~
WASHINGTON — Today (April 6 2023), the National Cattlemen’s Beef Association (NCBA) released a statement in regard to false information circulating on social media about the use of mRNA vaccines in cattle:
“There are no current mRNA vaccines licensed for use in beef cattle in the United States. Cattle farmers and ranchers do vaccinate cattle to treat and prevent many diseases, but presently none of these vaccines include mRNA technology.”
The National Cattlemen’s Beef Association (NCBA) has represented America’s cattle producers since 1898, preserving the heritage and strength of the industry through education and public policy. As the largest association of cattle producers, NCBA works to create new markets and increase demand for beef. Efforts are made possible through membership contributions. To join, contact NCBA at 1-866-BEEF-USA or membership@beef.org.
Shared from https://www.morningagclips.com/ncba-statement-correcting-internet-falsehoods-about-mrna-vaccines-in-cattle/
NCBA Slams FDA Commissioner’s Comments on Cell Cultured Meat
| MARCH 29, 2023
WASHINGTON (March 29, 2023) – Today, National Cattlemen’s Beef Association (NCBA) Vice President of Government Affairs Ethan Lane slammed Food and Drug Administration (FDA) Commissioner Robert Califf’s comments regarding cell cultured meat that he made during a hearing on the FDA’s fiscal year 2024 budget request:
“By his own admission, the FDA’s role is to ensure food safety, but Commissioner Califf’s comments today indicate that he intends to bring his agency into climate and environmental discussions while promoting cell cultured meat. This viewpoint is extremely disappointing to America’s cattle producers whose stewardship of the land already does more to protect our environment than fake meat production ever will. We appreciate Congresswoman Letlow shining a light on these concerning issues at FDA and hope that Commissioner Califf will reverse course and coordinate with the U.S. Department of Agriculture on the regulation of these cell cultured substitutes.”
Background
Today, FDA Commissioner Robert Califf testified before the House Appropriations Subcommittee on Agriculture, Rural Development, Food and Drug Administration, and Related Agencies. During the hearing, Rep. Julia Letlow (R-LA) asked the Commissioner how the agency plans to coordinate with the U.S. Department of Agriculture (USDA) on pre-market consultation for reviewing cell cultured chicken products. In his answer, Commissioner Califf referenced climate change and the need for additional cell cultured research as a way to mitigate the impact of climate change.
Under a memorandum of understanding signed in 2019, USDA and FDA have joint jurisdiction over fake meat products, with USDA taking the lead on enforcing accurate labeling and food safety. This memorandum was supported by NCBA because of USDA’s expertise in food inspections and labeling.
When FDA announced its second pre-market consultation for cell cultured chicken last week, the agency said that it is “ready to work with additional firms that are developing cultured animal cell food” and “will issue guidance to assist firms that intend to produce human food made from cultured animal cells.” These statements are highly concerning and indicate FDA’s desire to promote additional cell cultured meat products.
Learn More About American Agriculture: Search this page for terms you’re interested in
https://www.morningagclips.com
Eat Your Vaccines
April 3, 2023 • by The Vigilant Fox [Thomas Renz Research mentioned at 1:54 in Greg Reese Video]
“I’ve got documents from the NIH – from 2002 – talking about integrating vaccines into foods,” announced attorney Tom Renz in an eye-opening interview with Dr. Naomi Wolf. “They’ve been working on integrating these [vaccines] into our food supply. They’ve been working on it for at least two decades.”
“Gates, the WHO, a ton of these universities: they’re all talking about including mRNA vaccinations as part of the food. They’re gonna modify the genes of these foods to make them mRNA vaccines,” he warned.
Missouri HB 1169 seeks to counter such an effort. It’s been described as “one of the most controversial bills in history,” but all it is – is a labelling bill. If a food product is a gene therapy product, you have every right to know. So, if this bill gets passed, it’s a major victory not just for our well-being — but also for discovery, too. The full two-page bill is available to read here. [or below]
Dr. Naomi Wolf & Lawyer Thomas Renz
[House Bill 1169 mentioned at 1:40 in Greg Reese Video]
Biotech lobbyists in Missouri opposed to the HB1169 Gene Therapy Disclosure & Informed Consent Bill ADMIT that GMO food like the type Bill Gates is pushing to manufacture in factories WILL alter your genetics – ARE THESE FOODS GOING TO BE THE NEXT mRNA VACCINES?
HB1169 in Missouri would require disclosure of any product that would produce impacts on the human body similar to a gene therapy drug as well as requiring informed consent disclosure to include all risks and benefits – including adverse events of special interest. The language of the bill can be found [above]. Naturally, big Pharma and their associates oppose disclosure and informed consent.
Quick clip!
Edible Vaccines by Thomas Renz PART 1
[I listened to part 2 on this topic. It did not talk about Edible Vaccines, therefor not included on this page.]
During official testimony in the Missouri House on the bill the lobbyists for BioTech and WashU (I believe) in Missouri went on record to admit that GMO foods do in fact impact people’s genetic code. They actually opposed the bill on the basis that this law would require them to admit that all of their GMO products ARE in fact impacting people’s genetic code. There was no discussion as to how substantial the impact was or what studies were being done to ensure these modifications were not causing long-term health effects.
This SHOCKING admission is critical in light of the Gates Foundation/WEF push to begin producing all meats and dairy products in factories. If a basic GMO food can cause modification of our genome what could an entirely manufactured food do? Further, do we trust the same people to ensure these products are safe and effective that told us the COVID vaccines would stay in the injection site, have minimal side effects, and prevent the spread of COVID despite admitting to the European Union that they never studied the vaccine’s ability to stem the spread of COVID?
Edible Vaccines was written in 2000 by William Langridge: [Document at 10 seconds into Greg Reese Video]
2013 Paper referred to: Saxena, J., Rawat, S. (2014). Edible Vaccines. In: Ravi, I., Baunthiyal, M., Saxena, J. (eds) Advances in Biotechnology. Springer, New Delhi. https://doi.org/10.1007/978-81-322-1554-7_12
Shared from https://tomrenz.substack.com/p/renz-missouri-house-testimony-biotech (& links)
Emerging trends of edible vaccine therapy for combating human diseases especially COVID‐19: Pros, cons, and future challenges (Published 2022)
The researchers are still doing efforts to develop an effective, reliable, and easily accessible vaccine candidate to protect against COVID‐19. As of the August 2020, nearly 30 conventional vaccines have been emerged in clinical trials, and more than 200 vaccines are in various development stages. Nowadays, plants are also considered as a potential source for the production of monoclonal antibodies, vaccines, drugs, immunomodulatory proteins, as well as used as bioreactors or factories for their bulk production. The scientific evidences enlighten that plants are the rich source of oral vaccines, which can be given either by eating the edible parts of plants and/or by oral administration of highly refined proteins. The use of plant‐based edible vaccines is an emerging trend as it possesses minimum or no side effects compared with synthetic vaccines. This review article gives insights into different types of vaccines, the use of edible vaccines, advantages of edible vaccines over conventional vaccines, and mechanism of action of edible vaccines. This review article also focuses on the applications of edible vaccines in wide‐range of human diseases especially against COVID‐19 with emphasis on future perspectives of the use of edible vaccines.
Read full document:
Shared from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9347755/
FDA okays genome-edited beef cattle trait after safety review
Agency’s first enforcement discretion decision for an intentional genomic alteration in an animal for food use.
May 7, 2022 [Discussed at 2:06 in Greg Reese Video]
Today, the U.S. Food and Drug Administration announced it has made a low-risk determination for the marketing of products, including food, from two genome-edited beef cattle and their offspring after determining that the intentional genomic alteration (IGA) does not raise any safety concerns (low-risk determination). The IGA results in the equivalent genotype (genetic make-up) and short-hair coat trait seen in some conventionally bred cattle, known as a “slick” coat. This is the FDA’s first low-risk determination for enforcement discretion for an IGA in an animal for food use.
“Today’s decision underscores our commitment to using a risk and science-based, data-driven process that focuses on safety to the animals containing intentional genomic alterations and safety to the people who eat the food produced by these animals,” said Steven Solomon, D.V.M., M.P.H., director of the FDA’s Center for Veterinary Medicine. “It also demonstrates our ability to identify low-risk IGAs that don’t raise concerns about safety, when used for food production. We expect that our decision will encourage other developers to bring animal biotechnology products forward for the FDA’s risk determination in this rapidly developing field, paving the way for animals containing low-risk IGAs to more efficiently reach the marketplace.”
Based on the agency’s review of scientific data, the FDA has determined that the product is low-risk and does not raise any safety concerns, and the FDA does not expect the product developer of the IGA to pursue the FDA’s approval prior to marketing (enforcement discretion). To date, the FDA has made low-risk determinations for enforcement discretion for many other IGAs in animals for non-food uses and also has approved applications for five IGAs: in groups of goatExternal Link Disclaimer, chickenExternal Link Disclaimer, salmonExternal Link Disclaimer, rabbit and, most recently, in a line of pigs.
IGAs are alterations made using molecular technologies that introduce changes to the genome of an animal. The IGA in these cattle, known as PRLR-SLICK cattle, was introduced using a genome-editing technique known as CRISPR. The IGA can be passed on to offspring, allowing the trait to be shared through conventional breeding. There are conventionally bred cattle with naturally-occurring mutations that result in the same extremely short, slick-hair coat. Reports in scientific literature indicate that cattle with this extremely short, slick-hair coat are potentially able to better withstand hot weather. Cattle that are comfortable in their environment are less likely to experience temperature-related stress and may result in improved food production. Although PRLR-SLICK cattle have an equivalent trait to those cattle with a naturally-occurring short hair coat, they are not currently in commerce. The product developer plans to use the genetic products from these two animals with select customers in the global market soon and anticipates meat products will be available for purchase by general consumers as early as two years.
The FDA reviewed genomic data and other information submitted by the product developer confirming that the IGA in genome-edited PRLR-SLICK cattle is equivalent to naturally occurring mutations that have arisen in several breeds of cattle as an adaptation to being raised in tropical or subtropical environments. The data also confirmed that the IGA results in the same slick-hair trait as in cattle found in conventional agriculture. Further, the food from the cattle is the same as food from conventionally bred cattle that have the same slick-hair trait.
The FDA does not expect farms or facilities not owned or operated by the developer that are producing and breeding these low-risk PRLR-SLICK cattle using conventional breeding techniques to register with the agency. The low-risk determination was provided to Acceligen.
Shared from https://www.beefmagazine.com/news/fda-okays-genome-edited-beef-cattle-trait-after-safety-review
UK Parliament – Genetic Technology (Precision Breeding) Act 2023 (c. 6)
Passed: March 2023 [Document at 2:10 in Greg Reese Video] | 2022 Version here
Shared from https://bills.parliament.uk/bills/3167
Genetic Technologies (Precision Breeding) Bill | Commentary by Dr Pete Mills, Assistant Director, Nuffield Council on Bioethics 15 June 2022 [Discussed at 2:10 in Greg Reese Video]
The Bill
The precision breeding Bill is a kind of promissory note. Most of the detail remains to be determined, at some time in the future, through Regulations. What is on offer is really a framework for the governance of ‘precision bred organisms’ (the preferred rubric for a certain subset of genome edited organisms, those that are indistinguishable from organisms that might have come about through more established breeding methods). While Defra have indicated their intention to review the governance of all products of ‘modern biotechnologies’, the long title and provisions of this Bill do not allow its extension to transgenic GMOs – those containing DNA sequences derived from other species – or more creatively edited organisms. It is no doubt a prudent tactic to focus on securing more modest aims, though this may turn out to be at the expense of reinforcing the moral distinctness of the different classes of technology. This could be counterproductive: the different techniques generally have different ranges of potential application while, for some purposes, different techniques might need to be used in conjunction. Quick wins for genome editing may come at the cost of a prolonging the path for transgenic technologies rather than drawing them along in their slipstream. This is difficult to predict.
So what does the framework offer? [Finish reading this document]
More Reading https://www.nuffieldbioethics.org/publications/genome-editing-and-farmed-animals
The Genetic Technology Bill Is Not Fit For Purpose
Pat Thomas September 26, 2022
[Discussed at 2:10 in Greg Reese Video]
The Genetic Technology (Precision Breeding) Bill is set to return to Parliament soon to complete its Commons phase. The bill, which removes most regulatory controls from gene edited crops and foods in England, is making swift progress but is mired in controversy.
As drafted, it has raised multiple concerns about its scope, about the legality of some of its provisions, and, given public demand to see genetically engineered food regulated and labelled, about democracy.
The government has, nevertheless, pursued its deregulatory agenda with relentless energy, oblivious to anything beyond “deliver, deliver, deliver”. It has brooked no criticism, addressed no questions, and, thanks to its still healthy majority, strong-armed the bill through its early stages unamended. All of which gives the impression that everything is fine. Nothing to see here. Move along.
Except that’s not true. Storm clouds are brewing and government intransigence means it is increasingly falling to those outside of government to raise issues of legitimate concern.
Momentum behind these concerns is growing.
“Not fit for purpose”
In June, the Regulatory Policy Committee, for the second time, rejected the government’s business case for the bill, as laid out in its impact assessment (IA). The RPC, the independent better regulation watchdog, has branded the IA ”weak” and “not fit for purpose”. It notes that the government:
- has not adequately considered the full range of potential impacts arising from its invention of a new kind of GMO – the so-called “precision bred organism”
- has not sufficiently considered the full range of impacts upon small and medium businesses
- has not made a detailed assessment of the competition, innovation, consumer and environmental impacts
- needs to be clearer about the impact of removing labelling and traceability requirements
- needs to revisit assumptions relating to the impact on devolved administrations
It also cautions that most of the evidence regarding risk “is drawn from interested parties, or based on scientific trials, that do not replicate real-world conditions”. Such a narrative could, it says, “impede research, development and evaluation of an important new technology.”
A need to make amends
Also in June, a coalition of 36 UK civil society groups published a joint statement that cautioned: “We are concerned that too few MPs have grasped the full implications of the bill and that, as a result, it could pass into law without the full debate and major revisions it requires. We urge our parliamentarians to take steps to prevent this from happening.”
Thus far, no such steps have been taken and it remains unclear how many MPs who are debating and voting on the bill have even read it.
“Crucially, the bill proposes to remove all requirements of traceability, including labelling, from these technologies. If it passes in its current form, no-one—including farmers, businesses and citizens — will be able to exercise the right to choose whether or not to use, purchase or consume the products of these technologies. All surveys, polls and consultations show that people and businesses in the UK — whether or not they are supportive of agricultural genetic technologies — believe these technologies and their products should be regulated, traceable and labelled.
https://beyond-gm.org/wp-content/uploads/2022/06/GE-Bill_Civil-Society-Statement_100622_V6final.pdf
Environmental issues
If they had, they might be surprised to find the bill does not simply remove regulatory control from “precision bred” plants and animals but, through clever word play, removes such control from nearly all types of genetically engineered crops and foods, including those resulting from the insertion of foreign genetic material. Its scope is also not strictly limited to agriculture but quietly extends to wild and free-living flora and fauna which could also be “precision bred” without any formal assessment or post-release monitoring.
This is an ethical concern. It is also an environmental one.
Crucially, although the bill claims, in its very first paragraphs, that its provisions “will not have the effect of reducing the level of environmental protection provided for by any existing environmental law”, those provisions also require no environmental assessment or monitoring that might ensure the truth of that statement.
Censure from the scientific community
This month, several more concerns have been raised.
A joint statement signed by 90 international scientists and policy experts has criticised the government for the use of what is essentially a marketing slogan – “precision breeding” – in the bill’s title and text.
The statement contends the term is “technically and scientifically inaccurate” and “misleads Parliament, regulators, and the public” and should be deleted from the bill.
In conclusion, the term “precision breeding” should be deleted from the title of the UK government’s bill and replaced with terminology that is accurate and purely descriptive, to form a title such as “Genetic Modification Technologies (Food, Feed and Agriculture) Bill”. Beyond the context of this particular bill, governments and regulators worldwide should avoid using marketing terms such as “precision breeding” to describe gene editing and instead use scientifically and technically accurate terms with broadly agreed definitions, such as gene or genome editing, genetic modification, and genetic engineering.
https://docs.google.com/document/d/1bTXTWZwwDHfReRaiA4Kt25Jfrqab4iNyAlLAsEGTPR4/edit
London-based molecular geneticist Dr Michael Antoniou, who helped coordinate the letter, explains that the term “precision breeding” is being used:
“to give gene editing the appearance of controllability, predictability, familiarity, and therefore safety, implying that biosafety controls can be loosened or abolished”, something the growing number of signatories consider to be a “dangerous development”.
https://docs.google.com/document/d/1NMye5n0Q5Db5_n99LutYb9jXXSigLiFF/edit
Issues of commerce and trade
Echoing the RPC’s concerns, the organic sector has just published a joint letter to Secretary of State for the Environment, Ranil Jayawardena, raising the as yet unaddressed issue of co-existence, and the need for traceability throughout the food chain to ensure organic and non-GM farms and businesses can maintain their values-based and commercially valuable ‘GMO free’ status.
Concern about the bill has also been raised in a joint letter by civil society groups in the UK and EU which alleges the bill breaches the non-regression clause of the UK-EU Trade and Cooperation Agreement and asks the European Commission to take action. At the same time Scotland and Wales have affirmed they will not grow England’s gene edited crops or sell its gene edited foods, while Northern Ireland farmers and retailers, aligned with EU rules, cannot legally grow or sell them. No surprise then that a recent poll in New Scientist found that no supermarket was willing to say it would stock gene edited food. All of which suggests the RPC’s judgement is spot on.
A bill full of holes
Objections to the Genetic Technology Bill are not merely ideological but arise from the fact that it is full of holes, vague language and half-baked ideas.
It takes an issue overflowing with complexity, one that extends well beyond the narrow tramlines of laboratory science into ethics, environment, animal welfare, consumer choice, food culture, food sovereignty, equity and transparency, and abandons all the tricky detail to a future time and possibly a future government.
Many of its proposed regulations have been left to undebated ‘negative procedure’ secondary legislation. Even those under the ‘affirmative procedure’, which in theory can be debated, will, as the Secondary Legislation Scrutiny Committee points out, not benefit from any meaningful debate.
Finding – or forcing – a place?
In his new book, The Genetic Age: Our Perilous Quest to Edit Life, renowned popular science writer and Professor of Zoology at the University of Manchester, Mathew Cobb, concludes that while genetically engineered crops may well be safe, they have utterly failed to fulfil their promises of higher yields, lower pesticide use, improved biodiversity, better nutrition and, crucially, public buy-in.
This is largely a failure of the science itself. Hiding behind marketing slogans and removing regulation is not the way to help genetic technologies fulfil that promise. Indeed, given how much we still don’t know about genetics, it may bring unforeseen risks.
Cobb goes so far as to suggest that a single-minded focus on genetic technologies as THE solution is crude, ignores the complexity and interconnectedness of the problems we face and can draw attention away from other effective and less controversial solutions.
Environmental collapse, a global pandemic, climate change, war and economic downturn have sharpened our focus on the challenges that we are facing in producing food that doesn’t cost the earth. The fragility of our farming and food systems leaves no room for hype, no room for overblown promises and no room for grand experiments.
We have a responsibility, as critics of the bill are clearly saying, to ensure that any technologies intended to “fix the food system” are subject to high standards and clear criteria, not just scientifically but ethically, socially, environmentally and economically.
It’s a high bar, but if government truly believes in the transformative nature of agricultural genetic engineering then that is where the bar needs to be set – even if it means pausing the bill, or starting again – because real innovation always involves real risk.
Pat Thomas is a Founder/Director of Beyond GM/A Bigger Conversation
Shared from https://reaction.life/the-genetic-technology-bill-is-not-fit-for-purpose/
A sleight mistake: Magic goes wrong in the new Precision Breeding Bill
September 14, 2022 Julian Hitchcock Author [Discussed at 2:10 in Greg Reese Video]
In May, I set out my off-the-cuff reaction to what the Queen’s Speech had to say about an impending Genetic Technology (Precision Breeding) Bill, which was so general that it seemed merely to promise to duplicate regulations that had come into force the previous month on the release of genetically edited plants. The speech promised that the eventual Act would one day include animals, but otherwise it looked much the same. When the Bill emerged a fortnight later, it was swaddled in the inevitable boosterish blurb accompanying all government announcements on genetic technology. The media coverage was no less fevered. “Frankenfoods could be on supermarket shelves in the UK as soon as NEXT YEAR” the Mail needled, while the Telegraph stated that the Bill’s real aim is “to help guarantee British food supplies in the wake of the conflict in Ukraine”.
Genetics and brexit
We should not be surprised by this sort of political trumpeting. Genetics and politics have a notoriously troubled relationship, and the Bill is a child of a bitter political divorce. Its inspiration, an unintended gift to Brexiters from the EU Court of Justice (CJEU), was a decision so duff it seemed to confirm their claims about EU idiocy, scientific illiteracy and the opportunity to do things better. The UK’s advisory panel on the governance of synthetic biology, of which I was then a member, was summoned to Victoria Street to consider a UK response. We did not agree among ourselves[1], but by consensus recommended a change in the law on the basis that EU rules on genetic technologies had become absurd, people had voted to make their own rules in the 2016 referendum, and England and Wales now had the power to do so[2]: i.e. that it was possible. In his first speech as Prime Minister a year later, Boris Johnson boasted that the power to make British genetic technology regulations was a clear example of the advantage of Brexit. Many Remainers, including distinguished scientists and science journalists, reluctantly agreed.
The 2018 CJEU decision that lead to this, Confédération Paysanne, was absurd, but not because organisms produced using gene editing technologies such as CRISPR were deemed to be GMOs. That should never have come as a surprise: you edit a gene and you modify it genetically. What was startling was that the Court held (against the advice of its own Advocate General) that despite the clear words of the GMO Directive in question, an exemption specific to organisms derived by mutagenesis did not apply to those derived by precision mutagenesis (i.e. genome edited organisms). By contrast, GMOs derived by random mutagenesis were exempt from the bureaucratic palaver required by the Directive. The result was not only eye-swivelling, but grated against the EU’s own sustainability goals: you don’t have to go far to hear about the potential of genetic technologies like base and prime editing to meet global challenges such as food security, energy production and climate change.
Confédération Paysanne caused a stink, not just because the Court had bent law, policy and common sense into a hairpin, but for its rationale: a galumphing scientific gaffe. The justices treated two radically different methods of genetic modification as equivalent. The first is the one that lead to the Directive in the first place: taking a DNA sequence from one organism and transferring it into another: for example, inserting bacterial genes conferring glyphosate resistance into soybean genomes, or transferring human genes into mouse genomes to study disease. This transfer of DNA from one species to another is known as transgenesis. Transgenesis became controversial after a graduate student told a meeting in 1971 of her plan to splice genes from a virus into that celebrity bacterial resident of our guts, E.coli. After someone mentioned that the virus in question, SV40, could induce cancer in hamsters, scientists organised a conference in Asilomar to draw up biosafety rules. To cut a long story short, these rules morphed into today’s EU Directives and British laws on the containment and release of such transgenic organisms.
The second type of modification is the one the Court was looking at: genome editing. It involved using a tool like CRISPR to prune DNA already present in an organism. This process is called cisgenesis, and nothing gets transferred at all: in fact, it’s equivalent to what happens in breeding. The CJEU treated it as exactly the same as transgenesis.
Such was the ire in response to Confédération Paysanne that the unique advantages of transgenesis were neglected in a campaign to protect the cisgenic potential of new genome editing tools. In this series of posts, I’ll show how this seems to have led, in the EU as well as in the UK, to an unhelpful polarisation of policy, with genome editing cast as hero and old-school transgenic approaches as something less. The title of the Precision Breeding Bill tells you that nothing has changed as regards the regulation of GMOs in the UK, other than to complete the reversal of Confédération Paysanne in England that had begun in the April plant regulations. As we will see, the foundations of the Bill comprise the cis-trans distinction itself. The way it keeps the two apart is pure magic. As a piece of conjuring, however, it ends up all Tommy Cooper. We’ll see quite how wonky things get in the Precision Breeding Magic Show, which you can reach via the link at the end of this warm up.
What the Bill does
The Precision Breeding Bill seeks to establish and govern a new regulatory class of organism, defined by reference to the process of their production: “Precision Bred Organisms” or “PBOs” and their gametes. PBOs may be either plant or animal[3]. Additional “health or welfare” provisions apply in the case of “precision bred” animals. The Bill’s first objective is to regulate the release of PBOs from the highly controlled environment in which they are developed and studied as (sotto voce) GMOs. This is essentially an expansion of the April plant regulations. The second aim of the Bill is to regulate the marketing of products consisting of, or including, PBOs or their gametes. Making available for sale in England PBO-containing products (whether living or as food on a plate or feed in a trough) is an act of PBO marketing.
The most striking feature of the Bill is that it is less a set of rules than a statement of intent. Rather than providing details in Schedules, the proposed Act would empower the government to introduce future regulations to flesh out the detail. Fifteen of these thirty regulation-making powers avoid Parliament’s involvement altogether, including the power to “make supplementary, incidental or consequential provision in connection with any provision of or made under this Act”. Such proclamation-making powers are named after the autocrat who most used them. Dr Michael Edenborough QC voiced his unease to the Public Bills Committee: “there is also, in essence, a Henry VIII clause tucked away in clause 42, which is incredibly widely drafted. Those clauses always give rise to concern because, basically, you can do what you like, when you like, with very little scrutiny.”
The framework itself provides two mandatory PBO notification requirements: one for release and one for marketing. In order to get a “marketing notice” endorsed, an applicant has to secure a “precision bred confirmation” from the Department for the Environment, Food and Rural Affairs (DEFRA). This, in turn, is subject to DEFRA receiving confirmation from ACRE that the candidate PBO has, indeed, been “precision bred”. This term, “precision bred”, is the foundation stone upon which the Bill is built, but its meaning is so questionable that I have devoted an entire Magic Show to it. For now, let’s briefly note a few downstream issues. For example, if the PBO is an animal an additional “animal marketing authorisation” is required, which is only granted if a “welfare advisory body” is satisfied by the applicant’s “animal welfare declaration”: a statement, backed up by a methodology and evidence, that the applicant “does not expect the health or welfare of the relevant animal or its qualifying progeny to be adversely affected by any precision bred trait”. The cis-trans distinction emerges in the meaning of “qualifying progeny”, but note how it happens. If an organism has inherited any “functional” genes from a transgenic organism, it won’t qualify. By contrast, there is no limit on the number of cisgenic interventions an organism may have accumulated in the course of its family history. Nor is account taken of the effects of genomic interventions appearing at a rate far higher than in traditional breeding, including genetic material which did not previously give rise to functional protein, but does so as a result of an accumulation of edits made over numerous generations. Have you noticed the wiggle room afforded by that word, “or”? If the state values health over welfare, it can still provide a confirmation. Nor is it clear whether declarations and authorisations apply by class or on a case-by-case. These things are arguable, but that’s a problem. Regulations that leave room for argument invariably favour deep pockets, which is not a good look for a Bill purporting to unleash new enterprise.
But hush, the Precision Breeding Magic Show is about to begin!
| In the following series of posts, presented as a series of tricks in a magic show, Julian questions the meaning of the central term of the Precision Breeding Bill and reaches some rather unexpected conclusions about the meaning of “precision bred”. A sleight mistake: Trick one A sleight mistake: Trick two A sleight mistake: Trick three A sleight mistake: Trick four A sleight mistake: Trick five A sleight mistake: Epilogue |
Shared from https://www.bristows.com/news/a-sleight-mistake-magic-goes-wrong-in-the-new-precision-breeding-bill/
Disbelief as “Green King” Gives Royal Assent to New Gene Breeding Technology (Precision Breeding Bill)
[Discussed at 2:10 in Greg Reese Video] By Julian Rose April 10, 2023
In one of the more shocking hypocrisies of this year so far, Charles III, King of England – considered to be a strong supporter of organic farming and environmental causes – has given his Royal Assent to a biotechnology ‘innovation’ which will provide an open book for UK firms to alter the genome of animals and plants, so as to create novel engineered species and biotech ‘foods’.
In taking this step, Charles has committed an open act of betrayal of all bona fide farmers, and particularly of organic farmers.
The Genetic Technology Precision Breeding Act 2023 was given the royal go ahead on 23rd March, 2023. *
This piece of legislation will, for the time being, be unique to the UK, as such animal and plant biotech deformations are not allowed in the EU and many other countries.
A secondary deception relates to the marketing of such novel recombinant DNA experiments.
The UK government has stated that no separate definition will be given to gene technology-engineered products, therefore no special labelling will be required.
The dark irony of King of England launching unlabelled biotech foods, animals and plants on citizens of his own country, is difficult to trump.
Charles is already in conflict with the constitution of his country by standing shoulder to shoulder with Klaus Schwab in promoting the World Economic Forum’s ‘Great Reset’. One of the main objectives of which is to render nation states obsolete and to centralise all power within the control of a small despotic elite, whose stated intention is to make all private property illegal and to re-engineer human beings into Transhuman cyborgs.
On May 6, 2023, at his coronation in London, Charles will be officially crowned monarch of the United Kingdom and its Commonwealth (colonies). A large empire.
As the centre piece of the coronation ceremony, Charles will swear ‘The Coronation Oath’, essentially pledging his allegiance to the people of Great Britain and to protecting the sovereignty of the country and its traditions.
If Charles does not break his relationship with the World Economic Forum before this point, he will be performing an act of treason. The implications of this are profound.
As yet, the British people have not woken up to their fate. But should the truth emerge of this singularly blatant hypocrisy, the future of the British monarchy will be dark indeed.
The UK is officially recognised as a ‘constitutional monarchy’. With an unrevoked Common Law constitution stretching back to the Magna Carta of 1215, the true political power lies with the people and not with parliament, something which has been largely hidden from public knowledge.
If there is to be a future king or queen, the country needs that person to exercise his/her right to stand up against the continual parliamentary usurpation of the people’s power.
The people need a monarch with some guts, some wisdom and a genuine respect for truth. Someone who will use his time-honoured constitutional powers to block anti-life legislation like The Genetic Technology Precision Breeding Act 2023; thus setting a proper precedent for Great Britain’s ‘first among equals’ to act like a real King.
*Please see this link for official UK government act. For short version scroll down to c.6, 2023 Chapter 6 https://www.legislation.gov.uk/ukpga/2023/6/pdfs/ukpga_20230006_en.pdf
Julian Rose is an early pioneer of UK organic farming, a writer and international activist.
He is co-founder of The Hardwick Alliance for Real Ecology https://hardwickalliance.org/ and President of the International Coalition to Protect the Polish Countryside. Julian is a strong defender of pro ecological and traditional small farmers and successfully led ‘The Campaign to Save Real Milk’ against two UK government’s attempts to ban it. To find out more and to learn about his books, visit www.julianrose.info
Bill Gates “Solution: Lab-Grown Meat Is Made of Cancer Cells. Would You Like It Rare or Medium?
USDA does not allow animal tumors to enter food chain. But lab-grown meat is made of tumor cells
First published on February 24, 2023 [Mentioned at 3:00 in Greg Reese Video]
According to Bill Gates and the World Economic Forum, ongoing global warming threatens to destroy humanity. Methane, coming from the belches and farts of cows, is a greenhouse gas (GHG). So, cows are a problem!
Fortunately, Bill Gates has a solution for us, explained in this video. We need to stop growing cattle and switch to lab-grown synthetic beef.
The World Economic Forum expects we will eat “synthetic meat” in 16 years. (the article below was written 4 years ago)

Bill Gates made sizable investments in “synthetic meat” manufacturers, expecting to turn a nice profit.

The CNBC article explains that “lab-grown meat,” that is, cell cultures grown in giant stainless vats, is not the same as “fake meat” made of soy or pea protein:
Vegetarians have long touted the ethical and environmental problems with meat production and consumption. Start-ups such as MosaMeat, JUST and Memphis Meats are tissue-engineering meat in a lab to allow people to enjoy being a carnivore without any of the environmental or ethical hang-ups.
Dubbed clean meat, the efforts are distinct from “fake meat,” like the soy protein “chicken” you can find in your grocery store today. Unlike Morningstar or Boca Burgers, clean meat really is meat; it just grows in a lab instead of being part of an animal.
Okay, but what kinds of cells is that lab meat grown from?
Lab-Grown “Meat” is Made of “Immortalized” Cancer Cells
This excellent Bloomberg article (paywall-free link) clarifies that all lab meat is grown as immortalized tumor cells. As the article explains, these same cells are used to produce traditional vaccines.
Thank the biotech revolution. Under the right conditions, animal cells can be grown in a petri dish, or even at scale in factories full of stainless-steel drums. For decades, companies such as Pfizer Inc. and Johnson & Johnsonhave cultured large volumes of cells to produce vaccines, monoclonal antibodies and other biotherapeutics. Now the idea is that we might as well eat these cells, too.
What are these cells?
The big honking asterisk is that normal meat cells don’t just keep dividing forever. To get the cell cultures to grow at rates big enough to power a business, several companies, including the Big Three, are quietly using what are called immortalized cells, something most people have never eaten intentionally. Immortalized cells are a staple of medical research, but they are, technically speaking, precancerous and can be, in some cases, fully cancerous.
The article puts a “human face” on some of these cell lines, for example, the “HeLa line” made from the cervical cancer of Henrietta Lacks:
That’s where immortalized cells come in. They’ve been used in medical research since the early 1950s, when the first and most famous immortal cell line—derived from the cervical cancer cells of a woman named Henrietta Lacks — was successfully grown in a lab.
The distinction between pre-cancerous and cancerous cells is relatively minor: cancerous cells, by definition, can float away from the tumor site, travel through the blood or lymph, and start a new tumor (metastases) in another location in the body.
The distinction is important for the clinical outcome of a patient with a newly discovered tumor but involves only a minor bio-cellular distinction.
Don’t worry: Prominent cancer researchers tell Bloomberg Businessweekthat because the cells aren’t human, it’s essentially impossible for people who eat them to get cancer from them, or for the precancerous or cancerous cells to replicate inside people at all. … And cow tumors sometimes wind up in store-bought ground chuck, too. [not true – tumors are NEVER allowed by USDA inspectors – see below – I.C] Of course, the facts might not matter much if ranchers or other players in the traditional meat industry felt threatened enough to declare a public-relations war. It’s all too easy to imagine misleading Fox News chyrons about chicken tumors and cancer burgers.
Not so misleading! The main problem of growing an endless “lab meat” supply is that normal tissue cells cannot endlessly replicate (see above). There is a limit on how many times they will divide.
Vaccine manufacturers already use such immortalized tumor cells to make some Covid vaccines and other vaccines:
Today, AstraZeneca Plc and J&J’s Covid-19 vaccines are grown using immortalized human kidney and retinal cells, respectively.
Thus, “lab meat” and “cell line” suppliers grow meat from tumor cells that are “immortalized”; in other words, their cells can endlessly replicate. This is why cancers never stop growing, after all!
Eat Just Inc. declined to comment for this story. Believer Meats Chief Scientific Officer Yaakov Nahmias says that his company uses immortalized cells in its cultured chicken and that his team has somehow, by means he says even they don’t understand, created immortalized cells that don’t share any genetic signatures with cancer cells.
Are you skeptical of the above? I am. Even Bloomberg author Joe Fassler, to his credit, doubted the above explanation and asked independent biologists who also did not believe Yaakov Nahmias’s BS:
(Two cell biologists I shared his comments with expressed skepticism.)
We can see that so far, all lab meat is made using endlessly-dividing tumor cells.
Our bodies’ immune systems are designed to kill off and fight such abnormal and cancerous cells. Thus, cancers only take hold when immune systems weaken or the cancer cells learn to avoid immune reactions.
Cells become immortal in human bodies all the time, by mutating to bypass senescence—and mutating some more to evade the immune system, which generally tries to kill off such mutants.
The lab meat companies plan to sell those kinds of solid tumor cells to us to eat.
Bon appetit!
USDA Inspectors Screen Out Cancers and Tumors in Animal Carcasses
Despite Bloomberg’s restrained and soothing language, cancers and tumors can never pass USDA meat inspections.

Clarifications
The above story applies to “lab-grown meat”: products made from immortalized animal tumor cells growing in vats.
The “plant substitute” meat replacements, such as Beyond Meat, are based on pea or soy protein. As such, they are NOT related to these lab-grown cancer cell projects. Those plant products are not “meat” in any sense.
In addition, fake news websites are screaming that lab-grown meat (made of tumor cells) will give us cancer. There is no evidence that lab-grown meat will facilitate cancers. There is also no evidence that lab-grown meat will NOT cause cancers. It is simply an unknown. The USDA has a reason to reject tumor-containing carcasses, however. It is best not to make unwarranted claims, and my substack is not about making up sensationalized stuff.
Now They Want to Sell Us Solid Tumor “Steaks”
- The Bloomberg article explains that all “lab meat” is made of tumor cells.
- At the same time, USDA regulations forbid tumors in meat supply for humans.
I am sure that one way or another, Bill Gates and the WEF will lobby for a change in rules so that they can sell us solid tumors as “lab-grown meat.”
At this point, I am torn: would I rather eat “ze bugz”, or “lab-grown tumor meat.” Or go vegetarian? A tough decision!
What would YOU choose?
Shared from https://www.globalresearch.ca/lab-grown-meat-made-cancer-cells-would-you-like-rare-medium/5809869
Dr. Ana Maria Mihalcea [mentioned at 3:23 in Greg Reese Report]
[Mike Adams mentions that the mRna is already being injected into cattle. Please do your own research regarding if/when that has started.]
Follow more research by Dr. Ana at
https://substack.com/profile/41754232-ana-maria-mihalcea-md-phd
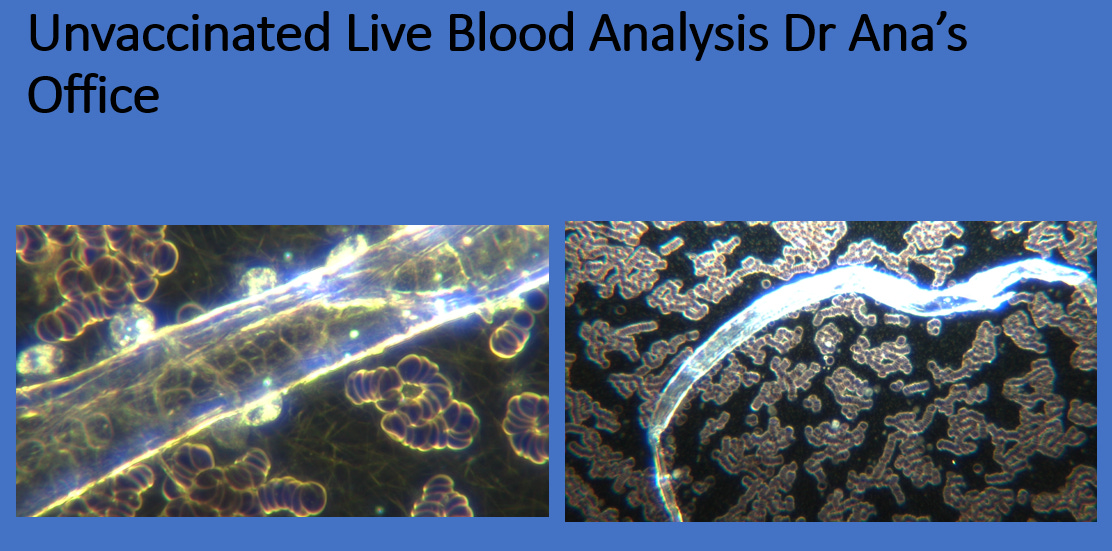
Contaminated Food Supply Contributing Cause To Live Blood Analysis Findings In Unvaccinated? Darkfield Blood Analysis On Grocery Meat Products
[mentioned at 3:45 in Greg Reese Report] April 2, 2023
A colleague of mine, Dr. David Jernigan treats vaccine injured patients successfully in Tennessee. He called me after I posted the results on Infrared Spectroscopy and electrical conductivity of live blood and confirmed all of my findings. He shared with me, that a short time ago some family members were eating meat and got very ill from it. Dr. Jernigan had developed a method to capture the frequency from a vaccinated rubbery clot of a deceased person and has developed a scientific way for detecting that frequency. He checked his ill family member, who tested positive. Then he checked the meat they ate and it had the same frequency of the cadaver blood clot. Subsequently he went to his local grocery store and checked meat products, and both organic and inorganic beef meats had the same frequency emission. He wanted me to go buy meat at my grocery store and do live blood analysis on different products to confirm his findings.
I have been wondering why I have been seeing every unvaccinated person in my office with contaminated blood. I have also seen an increasing amount of people with persistent severe diarrhea, that is lasting for months, but turns out to be negative when tested for ova and parasites or stool cultures for bacteria. I was suspecting vaccine shedding, chemtrail spraying, nasal swabs, masks, and contamination through synthetic biology named “ Covid and Long Covid”. Many are saying that what we are seeing in the blood are parasites, but they are not. They are self assembly hydrogel based synthetic life forms, more akin to microplastics but biologically engineered.
This morning I went to a local grocery store and bought different meats that had some visible blood in the package that I could analyze. I was also curious about milk products. Here are my results: [Follow source link below this article to view more images]
It appears to me that our food supply – in particular animal products – is contaminated with similar structures we find in human blood. Dr. Jernigan did frequency testing on fruits and vegetables and did not get a positive signal. I encourage others to do the same research and replicate my findings. This would explain to me why I see these live blood structures in everyone. It does not matter if the products are organic or not.
The next step is to find a local source and maybe do live blood analysis on cows or pigs. If they are contaminated even if unvaccinated, then this may be environmental, possibly via geoengineering spraying. The implications for humanity are profound. I asked my colleague Dr. David Nixon in Australia to replicate my findings, and we will ask Matt Taylor in Ecuador and Shimon Yanowitz in Israel. We need to verify these findings around the world. I will continue working with Dr. Jernigan.
Shared from https://anamihalceamdphd.substack.com/p/contaminated-food-supply-contributing
INTRODUCING GREENLIGHT Biosciences
An RNA platform to help feed the world and keep it healthy
[Completely WOKE Company]
Our partners: Some of our collaborators include the Bill and Melinda Gates Foundation, University of Wisconsin, UPL, and Bayer.
Sustainable food production through RNA solutions
We must grow more food with the same amount of land even as we honor the global desire—and increasing technical need—to replace chemical pesticides. While these pesticides face increasing consumer opposition and threat of outright bans due to environmental damage, many are losing their effectiveness.
VP of Plant Health R&D Ron Flannagan explains how GreenLight has demonstrated that RNA can be a viable solution to control six pests in our pipeline.
Rethinking our food supply
Our plant health business aims to leverage the GreenLight platform to empower farmers and the agriculture industry to grow food that is safe for people and the environment.
Where we are now
We’re just getting started.
GreenLight’s 13+ years of scientific progress has led us up to a few key successes.
Platform for mRNA
GreenLight’s platform, developed through 13 years of research and technology, is protected by foundational patents. Our process know-how, and the technology we developed to produce double-stranded RNA at metric-ton scale, can be leveraged and transformed, using our technical agility, for our mRNA platform.
7+ agricultural products in development
…with an addressable market of $6b that we plan to launch by 2026.
Demonstrated control of fungal pathogens
Control of fungal pathogens using double-stranded RNA has been demonstrated in field testing for the first time. This addresses the number one cause of food rotting. Based on this data, we have made the decision to move from discovery to development of a product to address the number one cause of food rotting. In the overall agricultural space, this represents an enormous market.
Progress for bees
Higher brood and health scores on bee colonies for our varroa mite treatment, compared to chemical controls with 2 and 8g/l application. The data from our trials to treats varroa mite infestation in beehives supports our progress toward commercialization and demonstrates that that we can acquire a product, improve upon it, and then move toward having a real-world solution that protects bees, beekeepers, and pollination-dependent crops.
A Covid-19 vaccine
We have witnessed promising antibody response and cell-mediated immunity for our GreenLight Covid-19 vaccine candidates in mice. The data indicates feasibility of initiating clinical trials in Africa for our vaccine candidate.
Bill & Melinda Gates milestone reached
Our RNA-based candidate is designed to deliver a healthy copy of the gene to stem cells. Our concept of simple injections of mRNA/lipid nanoparticle formulations is a treatment method we are actively researching with a Bill and Melinda Gates Foundation grant to develop gene therapies to treat sickle cell anemia. It’s time to move to the next phase of research.
To find out more, click here. (2021 Merger information)
~~~~
Nature is not an infinite resource, and we believe that with thoughtful research, development, and commercialization, we can create solutions that protect pollinators, support beneficial insect populations, and maintain soil health and water quality.
Our Plant Health pipeline stretches from protecting honeybees to fresh produce and large-scale crops, a $10 billion addressable market.
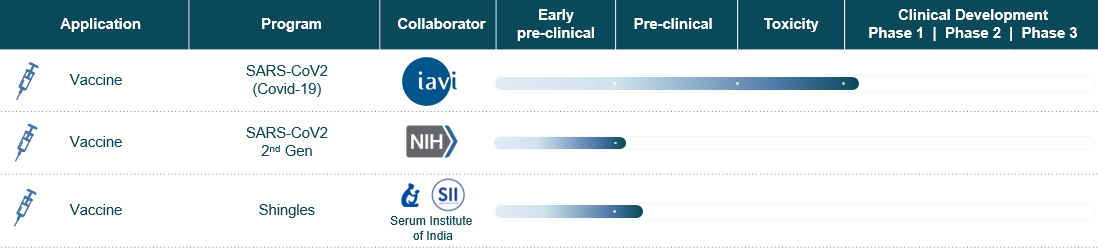
We have an expanding Human Health pipeline. Our design and manufacturing capabilities enable development of vaccines and therapies.
Messenger RNA for human health
Messenger RNA’s features make it broadly valuable for human health. It is well known that mRNA is being used to make effective Covid-19 vaccines that have been developed quickly—critical for pandemic response. GreenLight is working on multiple vaccine and therapeutic uses of mRNA.
GreenLight Biosciences Outlines Development Strategy and Highlights Portfolio Updates at Human Health R&D Day
- Progressing human health pipeline conferring mRNA platform advantages for infectious disease and personalized oncology medicines; pursuing capital efficient strategy targeting unmet medical needs, in both the developed and lower- and middle-income countries (LMICs), pairing focused R&D spend with partnerships.
- Accelerating development of next-generation COVID vaccine candidate; decision to advance universal pan-sarbecovirus vaccine candidate in place of mono-valent Wuhan based antigen.
- Preclinical data on shingles vaccine candidate(s) showing high and durable antibody response, strong cellular T-cell response, and durable memory response.
- Collaboration with EpiVax therapeutics to develop personalized mRNA cancer vaccine candidates.
- R&D Day webcast to begin at 10:30 am ET today.
BOSTON, March 9, 2023 —GreenLight Biosciences (Nasdaq: GRNA), a public benefit corporation striving to deliver on the full potential of RNA to address some of the world’s toughest problems in human health and agriculture, will today outline its pipeline strategy and R&D progress for mRNA-based solutions during its Human Health R&D Day being held today, March 9, 2023 at 10:30 a.m. ET in Lexington, MA.
GreenLight today is sharing the key pillars of its human health strategy:
- Developing vaccines for infectious diseases, especially those addressing unmet medical needs in lower- and middle-income countries. Consistent with its public benefit corporation status, Greenlight is striving to support global, sustainable vaccine access and pandemic response readiness.
- Developing innovative products to address unmet medical needs in oncology and autoimmune diseases. This work has begun with the collaboration with EpiVax Therapeutics to co-develop personalized cancer vaccines.
- Continued advancement of GreenLight’s mRNA technology platform through innovations and to seek potential partnerships with pharmaceutical and biotechnology companies.
As previously announced, GreenLight received approval from the Rwanda Food and Drugs Authority (Rwanda FDA) to initiate a Phase I/II study of its GLB-COV2-043 vaccine booster candidate. However, given the global shift in the standard of care for COVID vaccination to the Wuhan/Omicron bivalent vaccine, and new availability of the bivalent vaccine in Rwanda, GreenLight has decided to proceed with its pan-sarbecovirus vaccine candidate instead of the GLB-COV2-043 (monovalent) vaccine candidate as originally planned. GreenLight is accelerating the development of its pan-sarbecovirus vaccine candidate that is potentially capable of broader coverage and predictive protection and plans to make a follow-up filing on this candidate to the Rwanda FDA and the Rwanda National Ethics Committee (RNEC). GreenLight will be working expeditiously and closely with its Rwandan partners to advance these efforts over the coming months and are starting conversations with potential partners in other countries that have expressed interest in supporting its development and clinical path for a broader pan-sarbecovirus vaccine. “We are excited to advance our pan-sarbecovirus vaccine candidate that may allow us to bring a next generation, innovative Covid-19 vaccine to market in Rwanda, and the rest of the world, earlier than we anticipated,” Andrey Zarur, CEO of GreenLight.
Regarding its shingles program, GreenLight is excited to share promising new pre-clinical data. GreenLight has selected a lead candidate to progress towards clinical development after evaluation of multiple antigen designs and formulations. Based on pre-clinical data, our lead pre-clinical candidate induced antibody levels and memory B and T cell responses in mouse in vivo studies similar to the current standard of care. In addition, the pre-clinical data showed that the lead candidate induced T cell responses in mouse in vivo studies stronger than the current standard of care. This pre-clinical candidate is advancing towards development candidate nomination. Serum Institute of India Private Limited will be responsible for the clinical development, manufacturing, and commercialization of the vaccine candidate in lower- and middle- income countries under its license agreement with GreenLight Biosciences. GreenLight retains the clinical development, manufacturing, and commercial rights in the developed world.
GreenLight and EpiVax Therapeutics are partnering to create a proprietary personalized cancer vaccine platform which combines GreenLight’s mRNA design and manufacturing expertise with EpiVax Therapeutic’s neoantigen discovery platform. “We continue to be excited about expediting the development of personalized cancer vaccines in collaboration with EpiVax Therapeutics.” said Kimberly Warren, Chief Business Officer of GreenLight Biosciences. “While our initial focus will be on bladder cancer, the 4th most common cancer in men, we plan on working to shape a pipeline of compelling personalized cancer vaccine candidates that will help build a future of more timely and accessible treatments.”
The human health R&D Day is the second in a series providing comprehensive updates on GreenLight’s R&D strategy and progress. The previous session, which focused on GreenLight’s Plant Health pipeline, was held on Tuesday, March 7, 2023.
A live webcast of the event will be available live and can be accessed via the Investors section of the Company’s website at www.greenlightbiosciences.com. Registration is available here: https://www.greenlightbiosciences.com/rdshowcase/ and a replay of both events will be available at https://investors.greenlightbio.com for approximately 30 days.
GreenLight Biosciences Outlines Development Strategy and Highlights Portfolio Updates at Plant Health R&D Day
BOSTON, March 7, 2023 —GreenLight Biosciences (Nasdaq: GRNA), a public benefit corporation striving to deliver on the full potential of RNA to address some of the world’s toughest problems in human health and agriculture, will today outline its pipeline strategy and R&D progress for dsRNA based crop protection and plant delivery innovations focused on addressing food security with innovative and sustainable solutions during its Plant Health R&D Day being held today, March 7th, 2023 at 10:30 a.m. ET in Research Triangle, North Carolina.
“GreenLight is taking an innovative approach to advancing novel double-stranded RNA (dsRNA) solutions for numerous agricultural applications with advantages in speed of discovery/development, cost, quality and delivery. We have advanced our company’s portfolio strategy, target product profiles, and delivery capability all to meet the global challenges we face in the quest to grow food sustainably.” said Andrey Zarur, CEO of GreenLight Biosciences. “We have done this all with an eye on expanding potential addressable markets for our product portfolio and a focus on the potential benefits that our sustainable technologies could have on society.”
Sustainable innovations that address emerging resistance, food security, and agricultural impacts on biodiversity
As a leader in RNA innovation for agriculture, the Company is building on its existing rapid design, development and iteration capabilities for dsRNA by expanding product applications and delivery capabilities. The technological progress to be shared in today’s showcase enables GreenLight Biosciences to work towards unlocking access to the broader global insecticides and fungicides market worth $39B1,2, and the global herbicides market valued at $35.72B3.
“Farmers need products that appropriately solve the challenges they face in-field, all while helping to improve the overall sustainability of agriculture,” said Mark Singleton, Chief Commercial Officer and General Manager of Plant Health. “GreenLight Biosciences is fully committed to delivering products that address some of the major challenges faced by farmers, such as increased pest resistance, tightening regulatory environments, and demands by consumers to reduce chemical loads in foods. We expect that our rapid dsRNA development and production capabilities, coupled with novel delivery technologies, will enable us to develop sustainable solutions across all crop inputs segments and help reduce agricultural impact on biodiversity.”
Plant Health Strategy & Portfolio Updates
Today, at GreenLight’s R&D Showcase, the Company will outline a long-term strategy to progress its Plant Health portfolio and highlight progress in its R&D pipeline that it anticipates will help farmers expand their suite of tools to preserve yield potential as threats from insects, disease, and weeds increase, including:
- Actively preparing for commercial launch prior to end of 2023 of our leading dsRNA solution, Calantha™, subject to regulatory approval
- The registration dossier for our RNA solution that targets the varroa destructor mite was submitted to the EPA in February 2023 and is now under review
- Expansion of dsRNA-based fungal, insecticide, and acaricide programs into multi-target applications
- Plant disease program data showing formulated dsRNA performance in-field providing control of grey mold and powdery mildew equivalent or better than conventional chemical standards at commercially relevant spray intervals
- Data demonstrating functional delivery of dsRNA into plants; showing decreased fusarium disease severity in lettuce seed treatment trials, and preliminary indications of herbicidal activity
“These highlights are representative of our robust dsRNA pipeline, delivery capability and our ongoing commitment to delivering on the potential of RNA to provide farmers with the tools they need to increase productivity, profitability, and sustainability in the face of climate events and increasing resistance to conventional crop protection products,” said Zarur. “We look forward to advancing these innovations to ensure that farmers have access to technology that benefits their production, and to help create healthy people and planet through RNA innovation.”
The Plant Health R&D Day is the first in a series providing comprehensive updates on GreenLight’s R&D strategy and progress. The next session will focus on the Company’s Human Health portfolio and will be hosted on Thursday, March 9th, 2023, at GreenLight Biosciences Headquarters in Lexington, MA.
A webcast of both events will be available live and can be accessed via the Investors section of the Company’s website at www.greenlightbiosciences.com. Registration is available here: https://www.greenlightbiosciences.com/rdshowcase/ and a replay of the webcasts will be available at https://investors.greenlightbio.com for approximately 30 days.
A copy of the presentation can be found here: https://investors.greenlightbio.com/static-files/bb34331c-447f-4180-9663-96ba5a227542
About GreenLight Biosciences
GreenLight Biosciences (Nasdaq: GRNA) aims to address some of the world’s biggest problems by delivering on the full potential of RNA for human health and agriculture. Our RNA platform allows us to research, design, and manufacture for human, animal, and plant health. In human health, this includes messenger RNA vaccines and therapeutics. In agriculture, this includes RNA to protect honeybees and a range of crops. The Company’s platform is protected by numerous patents. GreenLight’s human health product candidates are in the pre-clinical stage, and its product candidates for the agriculture market are in the early stages of development or regulatory review.
Availability of Other Information About GreenLight Biosciences
Investors and others should note that we communicate with our investors and the public using our website (www.greenlightbiosciences.com), the investor relations website (https://investors.greenlightbio.com/), and on social media (Twitter and LinkedIn), including but not limited to investor presentations and investor fact sheets, U.S. Securities and Exchange Commission filings, press releases, public conference calls and webcasts. The information that GreenLight posts on these channels and websites could be deemed to be material information. As a result, GreenLight encourages investors, the media, and others interested in GreenLight to review the information that is posted on these channels, including the investor relations website, on a regular basis. This list of channels may be updated from time to time on GreenLight’s investor relations website and may include additional social media channels. The contents of GreenLight’s website or these channels, or any other website that may be accessed from its website or these channels, shall not be deemed incorporated by reference in any filing under the Securities Act of 1933, as amended.
Forward Looking Statements
Certain statements in this press release may constitute “forward-looking statements” for purposes of the federal securities laws. Our forward-looking statements include, but are not limited to, statements regarding our or our management team’s expectations, hopes, beliefs, intentions or strategies regarding the future, including those relating to the success, cost and timing of our research and development activities in our plant and human health programs, the timing of regulatory submissions and approvals, our ability to commercialize our products, the acceptance of RNA-based technologies by regulators and the public, including the timing it takes to receive regulatory approval, the success, cost and timing of our clinical trials, including estimates regarding when patients will be enrolled, our ability to progress our candidates into the clinic, if at all, the timing to commence future clinical trials, our projected cash runway and our ability to obtain funding for our operations when needed. Forward-looking statements include statements relating to our management team’s expectations, hopes, beliefs, intentions or strategies regarding the future. In addition, any statements that refer to projections, forecasts or other characterizations of future events or circumstances, including any underlying assumptions, are forward-looking statements. The words “anticipate,” “believe,” “contemplate,” “continue,” “could,” “estimate,” “expect,” “intends,” “may,” “might,” “plan,” “possible,” “potential,” “predict,” “project,” “should,” “will,” “would” and similar expressions may identify forward-looking statements, but the absence of these words does not mean that a statement is not forward-looking. These forward-looking statements are based on current expectations and beliefs concerning future developments and their potential effects. There can be no assurance that future developments affecting us will be those that we have anticipated. These forward-looking statements involve a number of risks, uncertainties (some of which are beyond our control) or other assumptions that may cause actual results or performance to be materially different from those expressed or implied by these forward-looking statements. These risks and uncertainties include, but are not limited to, those factors described under the heading “Risk Factors” in the Company’s most recent Annual Report on Form 10-K filed with the SEC, as well as discussions of potential risks, uncertainties, and other important factors included in our Quarterly Reports on Form 10-Q, periodic filings on Form 8-K, and any of our future filings with the SEC. Should one or more of these risks or uncertainties materialize, or should any of our assumptions prove incorrect, actual results may vary in material respects from those projected in these forward-looking statements. Some of these risks and uncertainties may in the future be amplified by current macroeconomic conditions and the ongoing COVID-19 pandemic and there may be additional risks that we consider immaterial, or which are unknown. It is not possible to predict or identify all such risks. Our forward-looking statements only speak as of the date they are made, and we do not undertake any obligation to update or revise any forward-looking statements, whether as a result of new information, future events or otherwise, except as may be required under applicable securities laws. For additional information on GreenLight and potential risks associated with investing, please see our public filings at https://www.sec.gov/edgar/browse/?CIK=1822691&owner=exclude.
The Peoples are threatened by the warp speed Orchestration of a Pandemic Famine
22 Jan 2023 / Author: Dr Alexandra Henrion Caude. [Not In The Greg Reese Report]
[*Webmaster Note: This article is mainly focused on graphene family derivatives & Carbon Nanostructures in vaccines. The sourced comments are many. Therefore, the article was shared due to the research, and ability for the reader to click through to the sources]
Declaration of Intentions
Currently, in France, and in so many other countries, while waiting for hypothetical and miraculous anti-flu “mRNA vaccines”, farmers continue to euthanize tens of millions of poultry… so that they cannot die infected by the flu…
What the Fake is going on? When will the farming world revolt, once and for all?
In October 2022, a study entitled, “Promising strategy for developing mRNA-based universal influenza virus vaccine for human population, poultry, and pigs- focus on the bigger picture”, even raised the possibility of creating a universal mRNA vaccine for humans, pigs and poultry. Mama Mia! La Pandemia!!! [55]
Christiane Lambert of the FNSEA – the main farmers’ mafia union in France – recently declared that the “mRNA Vaccine” to protect poultry, allegedly, against bird flu, would be ready in June 2023 – while the Minister of Agriculture, Marc Fesneau, had announced it for the fall of 2023. [30] In fact, the “Vaccine” of Ceva Santé Animale, the leader in animal health (a way of saying) in France, is being tested until the spring of 2023 and, as it requires 9 months of industrial production, it will not be marketed until 2024. Will the second laboratory, the German Boehringer, be able to accelerate its research at a “speed of science” faster than the “speed of science” of Ceva Animal Health? Surprise, surprise!!!
Which flu, by the way? Ask Anthony Fauci, the serial-killer/liar, the AIDS and COVID Joker-Doctor of the Globalists and Banksters. For near half a century, this arch-criminal has been playing his deadly flute on the tune of the flu… in its primary meaning of “influenza”, of penetration, of infiltration, of insinuation, of corruption, of jabbing… The Vaccine religion, with its toxic needles – is a religion of the “Voodoo Child”. And what about the Covid religion with its toxic Spikes?
By the way, with or without Graphene – for the new chicken mRNA vaccines? Dansez la Vaccinade vers la Grande Hommelette Finale!
I have already expressed myself, extensively, on this very subject, in French, in my essay entitled “Is the H5N1 Avian Flu the next false Pandemic orchestrated by the Vaccinalist and Eugenicist Globalists? Or is it, rather, a mutant H5N1 with a mutinous Covid flavor?” – which is the first chapter of my sequence “Orchestration of a Pandemic Famine.” [44]
On the subject of bird flu, and for the sake of commemoration, with regard to the guide Mouammar Kadhafi, assassinated by the CIA-infiltrated “Hungarian Dwarf” (Sarkozy) and the Clinton clique – in 2011. Kadhafi declared, in September 2009, in front of the General Assembly of the United Nations – which he accused of being a windbag – that viruses exist only for the sale of vaccines by the multinational pharmaceutical companies. Mouammar Kadhafi, a great benevolent dreamer, even called, there, for free medicines! Mouammar Kadhafi knew, pertinently, that the pathogenic viruses exist only in the unbridled propaganda of the genocidal Vaccine Freaks of the Pharmacrathy . [45] At the time, he even played the mocking-bird – about the bird flu – by prophesying the emergence of a fish flu in the future!
And here we are: today, fishes are vaccinated against a multitude of different pathologies… while poultry, waiting for an mRNA vaccine, are sacrificed, by tens and hundreds of millions – on the altar of the orchestration of Hunger – in order to avoid dying, hypothetically, from the flu by the virtue of an excremented shower from contaminated (and PCR tested) wild geese flying over their captivity shelters – when they are lucky to know the smell of “outside”.
Today, my transparent and declared objective, for this fifth chapter of my sequence “Orchestration of a Pandemic Famine, is to highlight the orchestration, by the Globalists eugenicists, of a new vector of graphenization of populations. It is the vector of “vaccines” which are permanently injected into farm animals throughout their survival life – very often in concentration camps.
Back in 2008/2009, when, with my son Ananda, we were shooting (images) in the west and mid-west, of USA, for the preparation of a 5 hours DVD documentary – the Titanic Apicole – about the industrial persecution of the honey-bees and about the Colony Collapse Disorder, we filmed terrific sequences of cattle and chicken concentration camps in Colorado or Iowa. Even organic? Indeed, even organic!
Is it necessary to specify that if graphene is found in the food chain – in meat, in eggs, in milk, etc. – because of the “vaccination” campaigns of farm animals, it is because these animals are graphenized during their life? Just like human animals are – without their knowledge, and so without their “informed consent”.
As summarized in a recent study published in December 2022: [53] «The usage of nanotechnology in veterinary medicine (veterinary nanomedicine) is still in its infancy… There is an urgent need for more research addressing nano-medecine pharmaco or toxico-kinetics in various farm animals, allergic and toxic reactions because of heterogeneous materials and their shapes, formula, and doses, animal species, and other unknown issues.»
And this, at least, as long as there is a livestock sector, because, if the Peoples do not reinitialize, very quickly, the Men of Davos – the gang of the World Economic Forum that has, even, placed a string of its Barbie Dolls in a position of official authority – it will be very bad for the future of their agriculture and farm animals… and for their food sovereignty.
Klaus Schwab’s Great Reinitialization, or Great Narrative, is just a very viral, and extremely pathogenic, variant of the Tikun Olam syndrome – which has been spreading and contaminating the Peoples… for a pretty long time – If I may.
In their pathetic and hugely protected (with 5000 soldiers) theater in Davos, the Globalists are only repeating their endless demented threats against the Peoples… who, moreover, are becoming more and more aware of the vicious, pathological, eugenic, inhuman – and even extraterrestrial, as John Kerry points out – nature of this self-proclaimed entity guiding the World.
Today, this deadly and morbid sect, which is the World Economic Forum, lies in full decay – witness, for example, the resignation of one of its junkies, Jacinda Ardern, in charge of New Zealand… after having transformed this country into a scorched war zone – from a social point of view. I would even be inclined to imagine that the Banksters, who are strictly devoid of empathy, could consider, discreetly, to blow some circuit breakers, in a near future… especially since the virtual “cloud”, of the real and huge FTX/Sam the Fried Banker scandal, is hovering, slyly, in the purulent atmosphere of some psychopaths affiliated to the World Economic Forum – or just dancing the Zelensky tango.
mRNA “Vaccines” : a code name for nano-metallised and, more importantly, nano-graphenised “Vaccines”?
Today, the vaccine obsession is based on mRNA: no vaccine salvation without this nucleic messenger. Moreover, the medical obsession, not to use the term “therapeutic”, also relies heavily on mRNA… and the so-called “gene therapies”
With “mRNA Vaccines”, all surprises are possible. To be convinced of this, it is enough to listen to the criminal Albert Bourla – Pfizer’s CEO since 2019 – attempting to pour out, by voice, an insipid, inarticulate and incoherent soup of supposedly scientific goodies and propaganda. Even to the point of conceding publicly, on television, that it is obvious that Pfizer does not create its vaccines from a real and existing Covid virus – but just from a pseudo-virus, a synthetic virus, namely from genetic virtual sequences spat from a computer. [51] [52]
Even Fakypedia, to say, has not managed to discover a photograph conferring a minimal semblance of intelligence [50] to this individual who was placed at the head of Pfizer at the same time as the Davos Economic Forum, in January 2019, orchestrated, very secretly, the fake pandemic to come at the end of the year. It was high times for Stéphane Bancel, the French boss of ModeRNA (then in non-virtual bankrupt) who was invited to hear about the excellent news… for Pfizer, for Moderna, for AstraZeneca, etc, and for all the Banksters of the world… as this fake pandemic is “nothing” but a global coup d’état to crush all the Peoples economically, socially, culturally and, in fact, existentially.
In fact, two of the worst criminals in charge of the Covidian vaccine genocide of the Nations – of the Goyims – are veterinarians. Very convenient! Antony Fauci is a veterinarian who got involved in state eugenics (AIDS, Covid, etc), in the US administration, for more than four decades. As to Albert Bourla, he is, also, a veterinarian who joined Pfizer, in 1993, and who specialized in animal reproduction biotechnology – namely in the creation of animal chimeras, in the literal sense of the word, because they are not natural.
Even lettuces are concerned… and threatened! [26] Indeed, on September 16, 2021, the University of California Riverside published an article presenting its recent research on the creation of an mRNA “vaccine” vectored by edible and cultivated plants – in this case lettuces and spinach… in order to fight, allegedly, against the invisible CoqueVide/19. According to Juan Pablo Giraldo, in charge of this research, «Ideally, one plant could produce enough mRNA vaccine for one person».
Juan Pablo Giraldo is the author of many publications: he is an expert in nano-particles, nano-structures, nano-technologies, nano-bionics – in the field of plants. For example, in April 2020, he published a study entitled “Targeted delivery of nanomaterials with chemical cargoes in plants enabled by a biorecognition motif” [29]
What a spell on these lettuces! Think about it: it is about forcing plants to assimilate chemical cargoes by disguising them with carbon nanotubes – for example…
In addition to being an RNA specialist, Juan Pablo Giraldo has also published some studies on graphene family derivatives: “Targeted Carbon Nanostructures for Chemical and Gene Delivery to Plant Chloroplasts” [28], “Sulfolipid density dictates the extent of carbon nano-dot interaction with chloroplast membranes” [22] and “Polymethacrylamide and Carbon Composites that Grow, Strengthen, and Self-Repair using Ambient Carbon Dioxide Fixation” [27].
Is this a coincidence? Absolutely not. In one of these studies, Juan Pablo Giraldo states that, «We developed targeted carbon-based nanomaterials as tools for precise chemical delivery (carbon dots, CDs) and gene delivery platforms (single-walled carbon nanotubes, SWCNTs) to chloroplasts, key organelles involved in efforts to improve plant photosynthesis, assimilation of nutrients, and delivery of agrochemicals. »[28]
In this particular example – the creation of an mRNA “vaccine” vectorized by lettuce and spinach – it is very clear that the researcher in charge of this vaccine creation is a graphene expert who uses graphene quantum dots, or carbon nano-tubes, to vectorize various charges. Even if none of the articles written, by the mainstream press, mentions these graphene derivatives used, by Juan Pablo Giraldo, as vectors – they are, nevertheless.
In conclusion, it seems very reasonable to propose that the term “mRNA Vaccine” be a globalist and catch-all term which is coded to designate nano-particle metallized vaccines… and especially nano-graphenized – and nano-graphenizing vaccines.
And I will prove it throughout this essay – which is somewhat technical, admittedly. Nevertheless, today, it is essential to educate oneself if one does not want to consume, directly or indirectly, lettuces/vaccines stuffed with polymers based on carbon nanotubes or, more simply, meat (or eggs, or milk) coming from farms totally vaccinated/injected with graphene derivatives – in order to protect them, allegedly, against an incredible number of duly invented pathologies… In fact, against an ever increasing number of pathologies. Why? Because the Pharmaceutical Mafia intends to rake in hundreds of billions of dollars – on mRNA “vaccines”.
Biodegradable Dendrimer Nano-particles in the New mRNA “Vaccines”
On January 12, 2023, Tiba Biotech – a new start-up founded in 2016 by MIT – signed a partnership with CEPI [15] to evaluate the next-generation RNA vaccine platform technology to fight “Disease X.” For the record, CEPI is the Coalition for Epidemic Preparedness Innovations (CEPI), an organization sponsored and funded by the World Economic Forum and the Bill and Melinda Gates Foundation. In short, a partnership contract between mafiosi.
As a reminder. The development of the “Covid” vaccine was announced at the World Economic Forum in Davos, January 21/24, 2020, one week before the WHO officially launched a global public health emergency on January 30, 2020, when the number of “confirmed cases” – by invalid PCR – worldwide (excluding China) was 150 (including 6 in the US).
On January 18, 2023, in Davos, CNBC host Rebecca Quick praised Moderna’s CEO for developing the Covid vaccine… before the virus existed in the United States. [31] During the debate, CNBC host Rebecca Quick revealed that at a WEF breakfast/meeting during the January 21/24, 2020 Forum, Stephane Bancel told her that Moderna was already working on a “Covid/19” vaccine…
The expression “Covid/19” was officially introduced to the world on February 11, 2020 by the WHO clown, Tedros Adhanom Ghebreyesus.
The term “Covid/19” means, very simply, “Covid/AI” as AI stand for 19 in the alphabet. “Certificate Of Vaccination IDentity. Artificial Intelligence”.
According to their introduction. «Tiba Biotech is a preclinical stage biopharmaceutical company developing next-generation RNA vaccines and therapeutics based on a novel dendrimer nanoparticle delivery platform initially developed at the Massachusetts Institute of Technology and the Whitehead Institute for Biomedical Research. Tiba Biotech’s nanoparticle delivery platform, RNABL™, can safely enable large vaccine and therapeutic payloads, with relaxed cold-chain requirements and superior safety compared to existing RNA technologies, to provide protection against multiple human and animal diseases ». [15]
Wait a minute! Superior safety? Are there any safety issues with RNA injections developed “at the Speed of Science”?
In September 2022, Tiba Biotech signed a partnership with the state of New South Wales, Australia, to develop next-generation mRNA vaccines, in fast tracking processes [20], against new emerging animal diseases that threaten Australia’s livestock sector and food security – including an mRNA vaccine against foot-and-mouth disease. [16] [17]
In June 2022, Tiba Biotech signed a partnership with the Cummings School of Veterinary Medicine of Tufts University to develop a multi-antigen, mRNA vaccine that allegedly protects against Powassan encephalomyelitis – vectored by the same tick species that transmit Lyme Disease. [18]
According to their propaganda, the world needs better RNA delivery because, today, most RNA-based drugs rely on decades-old technology – lipid nanoparticles (LNPs) – to enable their delivery into the human body. « Lipid nanoparticles are delicate nanoscale shells made of positively charged amino lipids that encapsulate negatively charged RNA. However, studies have shown that these lipid nanoparticle components can cause unwanted and dose-limiting toxicity and inflammation. This is also a highly contested patent space where a small number of early innovators are able to demand high licensing fees that chill new applications. Tiba has developed a portfolio of issued patents and patent applications worldwide, ranging from novel dendrimer chemistries to nucleic acid design.»
In short, Tiba Biotech claims to be in a position to introduce a new class of fully synthetic biodegradable nanoparticles [35] that would overcome the limitations – and toxicity – of lipid nanoparticles.
With or without Graphene?
New mRNA “Vaccines” surfing on Dendrimers: with or without Graphene?
In view of the fact that the Authorities and Globalists are constantly trying to graphenize the populations, it is tempting to postulate, once again, the hypothesis that the term “mRNA vaccine functionalized with dendrimeric nanoparticles” is a catch-all term coded to designate metallic nanoparticle vaccines… and especially nano-graphenized vaccines
See, in French, the CINaM article entitled “At CINaM, many chemists are interested in the synthesis of molecules called dendrimers, as well as in their properties and biological applications in order to make them the drugs of the future”. [54]
Indeed, the scientific literature, of the last few years, present many studies on the synergy between graphene derivatives and dendrimers – namely on the realization of graphene/dendrimer chimeras… for medical applications.
In fact, as far back as 2013, a study entitled, “Colloidal drug delivery systems in vaccine delivery” states that: «Colloidal carriers (liposomes, niosomes, microspheres, proteosomes, virosomes and virus like particles (VLPs), antigen cochleates, dendrimers and carbon nanotubes) have been widely explored for vaccine delivery.» [37]
In fact, as far back as 2006, a study entitled, “Functional polymeric nanoparticles: an efficient and promising tool for active delivery of bioactives” states that: «Various types of functional nanosystems, such as carbon nanotubes, quantum dots, polymeric micelles, dendrimers, metallic nanoparticles, and liposomes, are being extensively explored. However, high tissue accumulation of nonbiodegradable nanoparticles has caused toxicity problems and rendered them as not-so-popular therapeutic and diagnostic systems. The toxicity and safety of nonbiodegradable nanoparticles are subject to future research.»[40]
Here are a few other studies, for example, some of which openly mention the creation of graphene/dendrimer chimeras for vaccine delivery:
“Recent Advances in Nanotechnology for the Treatment of Melanoma”. 2021. Nanotechnology offers many benefits that could improve the life expectancy of melanoma patients with very low side effects. This review aims to examine the latest advances in nanotechnology as an innovative strategy for the treatment of melanoma. In particular, the use of different types of nanoparticles, such as vesicles, polymers, metals, carbon nanotubes, dendrimers, solid lipids, microneedles, and their combination with immunotherapies and vaccines will be discussed. [38]
“Active targeted drug delivery for microbes using nano-carriers”. 2015. This general study focuses on the four main types of nano-vectors… for vaccines and other so-called “gene therapies”: dendrimers, carbon nanotubes, liposomes and nanoparticles. [39]
“Pharmaceutical nanotechnology: from the bench to the market”. January 2022. [12] Nanotechnology is considered a new and rapidly emerging area in the pharmaceutical and medicinal field. Nanoparticles, as drug delivery systems, impart several advantages concerning improved efficacy as well as reduced adverse drug reactions.
Different types of nanosystems have been fabricated including carbon nanotubes, paramagnetic nanoparticles, dendrimers, nanoemulsions, etc. Physicochemical properties of the starting materials and the selected method of preparation play a significant aspect in determining the shape and characteristics of the developed nanoparticles. Dispersion of preformed polymers, coacervation, polymerization, nano-spray drying and supercritical fluid technology are among the most extensively used techniques for the preparation of nanocarriers. Particle size, surface charge, surface hydrophobicity and drug release are the main factors affecting nanoparticles physical stability and biological performance of the incorporated drug. In clinical practice, many nanodrugs have been used for both diagnostic and therapeutic applications and are being investigated for various indications in clinical trials. Nanoparticles are used for the cure of kidney diseases, tuberculosis, skin conditions, Alzheimer’s disease, different types of cancer as well as preparation of COVID-19 vaccines.
“Graphene-dendritic polymer hybrids: synthesis, properties, and applications”. 2019. [32]
“Simple preparation of maltose-functionalized dendrimer/graphene quantum dots as a pH-sensitive biocompatible carrier for targeted delivery of doxorubicin”. 2020. [33] It is about the creation of chimeras with dendrimers and graphene quantum dots – to vectorize allopathic remedies. [34]
“Dendrimer functionalized carbon quantum dot for selective detection of breast cancer and gene therapy”. 2019. This involves the green synthesis of graphene quantum dots from lemon peels. The graphene quantum dots are then functionalized with dendrimers for a targeted transfection of genes in the case of breast cancers. [36]
There was a virtual workshop organized, in March 2021, by the CNRS of Toulouse, which is entitled “Graphene oxide and carbon nanotubes functionalized by phosphorus dendrons and dendrimers for applications in oncology”. [19] It was followed by a thesis of the same name, under the supervision of Anne-Marie Caminade, defended in March 2022. [24]
Bayer: mRNA “Vaccines” for Animal Health
Research on RNA injected into animals is not new. In 2008, for example, a study was published entitled “MicroRNA: Mechanism of gene regulation and application to livestock”.
In May 2016, BioNTech announced its partnership with Bayer to develop innovative new mRNA-based vaccines and treatments for animal health applications. [4]
BioNTech, in late December 2022, announced the release of a new “mRNA vaccine” [46] to protect against malaria. [46] Note that it is humans who are vaccinated – not mosquitoes.
This malaria vaccine campaign is an opportunity for BioNTech to present its new “BioNTainer” concept – vaccine production modules that can be disseminated throughout Africa. The first modules are scheduled to be installed in Rwanda by the end of December 2022. [47]
In November 2021, at the World Health Summit – which hosted 6,000 people from 120 countries – Stefan Oelrich, the president of Bayer’s Pharmaceuticals division, said that: «Ultimately the mRNA vaccines are an example for that cell and gene therapy. I always like to say, if we had surveyed two years ago in the public, ‘would you be willing to take gene or cell therapy and inject it into your body?,’ we would have probably had a 95% refusal rate. I think this pandemic has also opened many people’s eyes to innovation in the way that was maybe not possible before ». [5]
Bayer tried to minimize the damage of this untimely statement by saying that it was a slip of the tongue regarding the mention of gene therapy. Bayer did not dare to comment on the rest of Stefan Oelrich’s statements. [6]
It goes without saying that the term “therapy” must be understood in the sense of “saving the planet” – in the whining mode of the “World” Economic Forum – by eliminating a part of the population through genocidal “vaccine” campaigns.
It is a cell and gene “therapy” insofar as nanoparticles, and in particular those of the graphene family, are vectors of cell mutation – or cell death for those injected who do not survive.
It was the same Stefan Oelrich who, at the same summit – of medical and sanitary ineptitudes and corruptions – mentioned the mission of Bayer Pharmaceutical, as well as other leading institutions and personalities, in promoting contraception in developing countries. He even stated quite clearly: « We also need to focus on what is socially responsible outside of Europe and ensure sustainable action there. We pledged, this past year, to give an additional hundred million women access to contraception in the world. We’ve invested 400 million this year into new plants that are dedicated to produce long-acting contraceptives for women in low-and-middle income countries … Together with Bill and Melinda Gates we’re working very closely on family planning initiatives.»
In his speech in November 2021, Stefan Oelrich spoke of the “Bio Revolution” – a new fad promoted by the McKinsey firm… experts in all kinds of corruptions. This mafia organization titles, in fact, on its website: “The Bio Revolution: Innovations transforming economies, societies, and our lives”. [12]
According to its definition: «The Bio Revolution is a confluence of advances in biological science and accelerating development of computing, automation, and artificial intelligence is fueling a new wave of innovation. This Bio Revolution could have significant impact on economies and our lives, from health and agriculture to consumer goods, and energy and materials.»
Merck: mRNA “Vaccines” for Animal Health
In January 2019, Merck announced the launch of a revolutionary new RNA-based vaccine platform called “Sequivity” [23] [25] which is presented as “The support you need to meet ever-evolving swine disease threats head-on”.
According to their introduction: «Combat current and future swine diseases with SEQUIVITY from Merck Animal Health. A revolutionary swine vaccine platform, SEQUIVITY harnesses RNA particle technology to create customized prescription vaccines against strains of influenza A virus in swine, porcine circovirus (PCV), rotavirus and beyond. It’s supported by a sophisticated dashboard filled with comprehensive data and insights, all to help you stay on top.» [7]
According to their propaganda: «Traditional vaccines can take years to develop. SEQUIVITY’s RNA particle technology allows for the development of safe and flexible custom swine vaccines in only 8-12 weeks.»
This revolutionary new RNA-based vaccine platform generates a synthetic sequence that does not require manipulation of pathogen DNA/RNA. Its flexibility allows it to construct vaccines from multiple swine pathogens and/or A strains.
The same is true for the invisible Covid/19 which does not exist – except as virtual computerized genetic sequences.
As a reminder. It was Merck that, in November 2015, acquired Harris Vaccines, Inc – a privately held company that develops, manufactures and sells vaccines for livestock and companion animals. [9]
It is this firm, Harris Vaccines, which has been marketing an “mRNA” anti-influenza vaccine for pigs (H3N2 cluster IV) in two doses for several years [30], as well as an “mRNA” anti-flu vaccine for poultry. [42]
For these two injections, Harris Vaccines gives the following explicit precautions [49]: «Do not vaccinate within 21 days before slaughter. Freeze at – 80°C for long term storage. Use within 7 days if stored at 4°C. Use entire contents when first opened/punctured. In case of anaphylactic reactions, administer epinephrine ».
As we have seen with all the other anti-vaccine injections from the Pharmacratic Mafia, the extreme low storage temperatures – under the pretext of protecting the fragile RNA – are required so that the graphene does not start to flocculate under the effect of the ambient heat.
Question: What happens if the meat is sold immediately after vaccination? Spanish farmers, for example, have posted a video of a chicken, ready to be sold, whose body continued to pulsate under the effect of an unknown energy. Could it be the graphene oxide activated by a nearby energy source?
We learn, moreover, from Merck’s press release, dated November 12, 2015, that: «This pioneering system is rapidly adaptable to new disease challenges and was instrumental in producing the first conditionally licensed vaccine to help control Porcine Epidemic Diarrhea Virus (PEDv), a deadly virus that has killed more than eight million piglets since suddenly emerging in the U.S. in 2013. In September 2015, Harrisvaccines received a conditional approval for a Eurasian H5 subtype avian influenza vaccine and was subsequently awarded a contract by the United States Department of Agriculture’s Animal and Plant Health Inspection Service (USDA/APHIS) to produce the vaccine.» [9]
Is it clear that Merck declares, in all transparency, that an emergency and conditional authorization, by the USDA, allowed them to introduce, commercially, “mRNA vaccines”, as soon as 2014/2015, against, allegedly, the swine epidemic diarrhea virus. A first “mRNA vaccine”, anti-diarrheal, was officially approved in June 2014 [8] and a second “mRNA vaccine”, anti-flu, was authorized in September 2015.
Graphene and Chitosane in “Vaccines” for poultry
A recent study, published in December 2021, “Enhancement of antiviral activity of egg yolk antibodies against Chinese sacbrood virus” discusses the enhancement of antiviral activity of egg yolk antibodies against Chinese sacbrood virus through vaccination of poultry with graphene and chitosan-based injections. [164]
According to the introduction. Chinese sacbrood virus (CSBV) poses a serious threat to the apiculture of China. Although several approaches have been attempted to control CSBV infection, their applications have been greatly limited in practical breeding of honeybees due to poor effectiveness. Egg yolk antibodies (EYA) have shown a promising protection for bees against CSBV infection. This study was conducted to produce high titer EYA and then further improve their antiviral effect. Among three vaccination groups, the EYA titer in graphene oxide-chitosan group was highest (1.591 ± 0.145), in Freund’s group was modest (1.195 ± 0.040), and in white oil group was lowest (1.058 ± 0.056). After three injections of each vaccine in hens, EYA were produced at the highest level with a 14-day period. After application of EYA for more than two years in actual bee breeding, prevention and treatment assays showed that EYA confered 98.9 to 100% protection from CSBV infection. The mortality of the control group reached to a range of 91.2 to 100%. This study demonstrated that the high titer EYA have been successfully prepared with significant anti-CSBV activity and that these antibodies may feasibly be used for CSBV treatment to meet the practical needs of apiculture.
There is also a 2019 study entitled “Novel carbon quantum dots can serve as an excellent adjuvant for the gp85 protein vaccine against avian leukosis virus subgroup J in chickens”. [172] It is a vaccine, based on graphene quantum dots, against, allegedly, the avian sarcomatous leukosis virus.
Graphene in “Vaccines” for fishes
Do we need to specify that the fish “vaccine” industry has existed for about 50 years?
Do we need to specify that DNA-based “vaccine” for fish have existed since at least 2005? See the study “DNA vaccines for aquacultured fish”. [146]
Do we need to specify that nano-particles are already abundantly used in “vaccine” for fish. For example, PLGA. [154]
I found about ten studies which are about the inclusion of graphene derivatives in vaccines for fish.
“Recent Advances in Application of Nanoparticles in Fish Vaccine Delivery”. 2017. [155]
« There is a constant need for the development of efficient vaccines and delivery systems to prevent and control the emerging and re-emerging infectious diseases in aquaculture. There are innumerable infectious diseases for which the development of efficient vaccines has been difficult to achieve. The failure is mainly due to the inability to design vaccines evoking appropriate immune responses. The use of nanoparticles has provided a tremendous opportunity to design vaccine delivery systems that are efficient in targeted delivery, providing stability to antigens, and act as efficient adjuvants. Many of the nanoparticles are able to enter the antigen presenting cells by different pathways and induce appropriate immune responses to the antigen.
A number of different nanoparticles are used in fish vaccine delivery, which includes biodegradable polymers, nanoliposomes, carbon nanotubes, calcium phosphate, and immunostimulating complexes (ISCOMs), among which poly (lactic-co-glycolic acid) and chitosan are the most studied form of nanoparticles to date. Hence, the use and application of other forms of nanoparticles need to be explored. This review provides an overview of the use of different nanoparticle systems for the delivery of fish vaccines and compares the potential of these delivery systems for the development of new vaccines against different fish pathogens.» [155]
It is well stated in 2017 that these nanoparticles, for example of graphene, are already used for the administration of vaccines to fish.
“Immune efficacy of carbon nanotubes recombinant subunit vaccine against largemouth bass ulcerative syndrome virus”. 2020. This study investigates the immune efficacy of a recombinant carbon nanotube-based vaccine against the Large Mouth Archigan (Micropterus salmoides) ulcerative syndrome virus. [151]
According to their findings. Our results demonstrate that single-walled carbon nanotubes can be used as a novel method of ulcer immunization.
“Carbon nanotube-based DNA vaccine against koi herpesvirus given by intramuscular injection”. 2019. This study focuses on the efficacy of a carbon nanotube and DNA-based vaccine against a herpes virus in common carp. [158]
“Carbon nanotubes-loaded subunit vaccine can increase protective immunity against rhabdovirus infections of largemouth bass (Micropterus Salmoides)”. 2020. This study investigates the use of single-walled carbon nanotubes to allegedly vaccinate against a purported rhabdovirus in the largemouth bass (Micropterus Salmoides). [152]
“Application of Carbon Nanotubes in the Advancement of Fish Vaccine”. 2022. [159]
«Recently, nanodelivery system has been developed to improve the administration and efficacy of vaccines. Nanoparticles can be easily turned up to have specific chemical and physical characteristics. Likewise, carbon nanotubes (CNTs) are new alternative and efficient tool for transporting and translocating therapeutic molecules. As the functionalized CNTs are not immunogenic and have low toxicity, highly biocompatible, they hold tremendous potential in nanomedicine and nanobiotechnology. CNT-based drug delivery is promising for higher efficacy with lower side effects in achieving the higher effectiveness of drugs. CNTs are being utilized delivery vehicles for vaccines to protect farmed fish against disease-causing pathogens. This book chapter sheds the light on CNTs as a potential novel tool as vaccine carrier against various bacterial and viral diseases in fish. The importance of CNTs to enhance sustainable aquaculture has also been highlighted in this chapter.»
“Single-walled carbon nanotubes as delivery vehicles enhance the immunoprotective effect of an immersion DNA vaccine against infectious spleen and kidney necrosis virus in mandarin fish”. 2020. It is a vaccine based on carbon nanotubes and DNA intended to fight, allegedly, against a necrosis in the Chinese perch (Siniperca chuatsi). [142]
“Advances and Perspectives in the Use of Carbon Nanotubes in Vaccine Development”. 2021. [157]
«Advances in nanobiotechnology have allowed the utilization of nanotechnology through nanovaccines. Nanovaccines are powerful tools for enhancing the immunogenicity of a specific antigen and exhibit advantages over other adjuvant approaches, with features such as expanded stability, prolonged release, decreased immunotoxicity, and immunogenic selectivity. We introduce recent advances in carbon nanotubes (CNTs) to induce either a carrier effect as a nanoplatform or an immunostimulatory effect. Several studies of CNT-based nanovaccines revealed that due to the ability of CNTs to carry immunogenic molecules, they can act as nonclassical vaccines, a quality not possessed by vaccines with traditional formulations. Therefore, adapting and modifying the physicochemical properties of CNTs for use in vaccines may additionally enhance their efficacy in inducing a T cell-based immune response. Accordingly, the purpose of this study is to renew and awaken interest in and knowledge of the safe use of CNTs as adjuvants and carriers in vaccines. » [157]
“Single-walled carbon nanotubes as delivery vehicles enhance the immunoprotective effect of a DNA vaccine against spring viremia of carp virus in common carp”. 2017. It is a vaccine based on carbon nanotubes, single-walled, against, allegedly, a virus in carp. [153]
“Functionalized Multi-Wall Carbon Nanotubes Enhance Transfection and Expression Efficiency of Plasmid DNA in Fish Cells.” 2015. According to their findings: All these results indicated that carbon nanotube-based, NH₃⁺,single-walled complexes could be suitable for the development of a DNA vaccine for viral infection control in the aquaculture industry. [156]
“Applications of carbon nanotubes and polymeric micro‐/nanoparticles in fish vaccine delivery: progress and future perspectives”. 2021. [147]
Here is a recent video about the only Spanish unit of « vaccination », i.e. injections of poisons. [129]
“Optimizing the immunization procedure of single-walled carbon nanotubes based vaccine against grass carp reovirus for grass carp”. It is a vaccine based on single-walled carbon nanotubes, against, allegedly, a virus in carp. [162]
“Single-walled carbon nanotubes as candidate recombinant subunit vaccine carrier for immunization of grass carp against grass carp reovirus”. It is a vaccine based on single-walled carbon nanotubes, against, allegedly, a virus in carp. [166]
Graphene in “Vaccines” for cattle
There are a few studies on this specific issue.
“Immune response and biochemistry of calves immunized with rMSP1a ( Anaplasma marginale) using carbon nanotubes as carrier molecules”. 2018. This study focuses on the vaccination of calves against the bacterium Anaplasma marginale using vaccines concocted with multi-walled carbon nanotubes. [148]
“Efficient delivery of DNA into bovine preimplantation embryos by multiwall carbon nanotubes”. 2016. According to the abstract: « The pellucid zone (PZ) is a protective embryonic cells barrier against chemical, physical or biological substances. This put, usual transfection methods are not efficient for mammal oocytes and embryos as they are exclusively for somatic cells. Carbon nanotubes have emerged as a new method for gene delivery, and they can be an alternative for embryos transfection, however its ability to cross the PZ and mediated gene transfer is unknown. Our data confirm that multiwall carbon nanotubes (MWNTs) can cross the PZ and delivery of pDNA into in vitro-fertilized bovine embryos. The degeneration rate and the expression of genes associated to cell viability were not affected in embryos exposed to MWNTs. Those embryos, however, had lower cell number and higher apoptotic cell index, but this did not impair the embryonic development. This study shows the potential utility of the MWNT for the development of new method for delivery of DNA into bovine embryos.» [160]
“Balanced Th1/Th2 immune response induced by MSP1a functional motif coupled to multiwalled carbon nanotubes as anti-anaplasmosis vaccine in murine model”. 2019. Again, this is a cattle vaccine against Anaplasma, using multi-walled carbon nanotubes. [167]
“Evaluation of humoral and cellular immune response of BALB/c mice immunized with a recombinant fragment of MSP1a from Anaplasma marginale using carbon nanotubes as a carrier molecule”. 2014. Again, this is a cattle vaccine against Anaplasma, using multi-walled carbon nanotubes [168]
Need we point out, for example, that carbon nanotubes can enter the body of poultry through routes other than vaccination. For example, according to this 2019 study “Carbon nanotubes significantly enhance the biological activity of CpG ODN in chickens”, [163] carbon nanotubes are mixed with synthetic unmethylated cytidine-phosphate-guanosine oligodeoxynucleotides in order to, allegedly, stimulate the immune activity of poultry.
“IgY against enterotoxigenic Escherichia coli administered by hydrogel-carbon nanotubes composites to prevent neonatal diarrhoea in experimentally challenged piglets”. 2016. It is a vaccine against, allegedly, Escherichia coli in newborn pigs, which is concocted from a hydrogel composed of chitosan and carbon nano-tubes. [170]
Graphene in “Vaccines” for zoo animals
I have already reported on the post-vaccination deaths of several zoo animals who died following their Covid/19 injections. These injections are, of course, just as graphenized as those intended for human animals – which, by the way, were also confined in zoos, or concentration camps, during the most dictatorial episodes of the false pandemic.
It should be noted that as early as February 2021, ferret vaccination campaigns were organized with, allegedly, lightened versions of Pfizer and Moderna’s anti-Covid injections. Lightened in what way… since the mRNA is, very often, absent? Lightened in graphene oxide?
In fact, many zoo animals have officially died from Covid/19 when in fact they died from their graphenized vaccination. For example, three snow leopards died from CoqueVide/19 at a zoo in Illinois, USA. [11]
The same is true of a lion in a zoo in Africa that was vaccinated against Covid as part of a vaccination program that covered most animals in zoos, wildlife parks and reserves. [13] The sudden, “spiraling” death of this vaccinated lion was captured on video. [10]
There are many videos on the Web showing people (vaccinated Covid) who die suddenly after their bodies have made a round in the air. That is, an almost complete circular movement of their body – like a terminal dance movement. The death spiral has been observed in humans dying suddenly, but its appearance in animals is a new phenomenon.
In 2011, the company Zoetis (ex-Pfizer), based in New Jersey in the USA, sent 27,000 doses of its experimental vaccine to nearly 70 zoos, in the country, for 100 species of mammals. [14] [110] Thus, many animal species were vaccinated against the non-existent pandemic: gorillas, tigers, hippopotamuses, hyenas, lions, etc.
On June 22, 2022, Kimani, the young gorilla – from the Calgary Zoo in Canada – died of liver cancer. [1] 66 animals, from this zoo, had received their first dose of Covid injection, at the beginning of April 2022 – including two gorillas, a leopard, a tiger, etc… [2]
In May 2022, it was the female gorilla, Martha, who died of sudden death, coincidentally – as a result of her Covid injection. [3]
In November 2022, a study proposed a “mRNA vaccine” to curb the notorious disease, Rabies. “An mRNA-based rabies vaccine induces strong protective immune responses in mice and dogs”. [43]
As with all other farm animals, vaccines for zoo animals will be – and already are – “mRNA vaccine”… the ultimate Vaccinade.
This study, from February 2022, states it most clearly. “mRNA Vaccine Development for Emerging Animal and Zoonotic Diseases” [49].
« In the prevention and treatment of infectious diseases, mRNA vaccines hold great promise because of their low risk of insertional mutagenesis, high potency, accelerated development cycles, and potential for low-cost manufacture. In past years, several mRNA vaccines have entered clinical trials and have shown promise for offering solutions to combat emerging and re-emerging infectious diseases such as rabies, Zika, and influenza. Recently, the successful application of mRNA vaccines against COVID-19 has further validated the platform and opened the floodgates to mRNA vaccine’s potential in infectious disease prevention, especially in the veterinary field. In this review, we describe our current understanding of the mRNA vaccines and the technologies used for mRNA vaccine development. We also provide an overview of mRNA vaccines developed for animal infectious diseases and discuss directions and challenges for the future applications of this promising vaccine platform in the veterinary field.» [49].
On the part of these “scientists”, it is, purely and simply, propaganda and complicity in crimes against the People.
Moreover, contrary to one of the insanities uttered by these authors, it is not a virus that killed half of the 433 million pigs in China, in 2019: it is an extermination campaign impelled by the Chinese Bolshevik Party so that the pigs do not die, supposedly, contaminated.
Indeed, in China, the orders were to destroy all pigs within a radius of three kilometers … at the first suspicion of a case of African Swine Flu. In other words, it was a PsyOps orchestrated by the Chinese State in order to eliminate millions of small Chinese farmers.
Indeed, at that time, 40% of the pig production was still coming from small farms (less than 500 pigs) which numbered 2.6 million. As a reminder: the number of pigs slaughtered in China is 700 million annually, generating more than 50 million tons of meat.
Robert Malone mRNA vaccines for livestock, pets & wildlife
Jan 11 2023 [Not in the Greg Reese Report]
[*Webmaster Note: Posting the conclusion first, the research afterwards]
Conclusion: There were news stories in 2020 that mRNA vaccine(s) were being developed for COVID/SARS-CoV-2 for administration to livestock and companion animals. However, the lack of updates suggest that these plans may have been scrapped with the new, less virulent variants.
Before we can discus mRNA vaccines for livestock, pets and wildlife, we must first address the elephant in the room. That is, how come the public is able to access human clinical trial information, but is not able to do the same for clinical trials involving animal health?
During the early days of the AIDS epidemic, the AIDS community demanded public access to clinical trials. In 1988, the U.S. Congress passed the Health Omnibus Programs Extension Act of 1988 (Public Law 100-607) which mandated the development of a database of AIDS Clinical Trials Information Services. This Congressional Act motivated other non-profit disease related groups to demand access also.
The Food and Drug Administration Modernization Act of 1997 amended the Food, Drug and Cosmetic Act and the Public Health Service Act to require that the NIH create a publicly available clinical trials database. This eventually led to the development of the website ClinicalTrials.gov. This allowed tracking of drug efficacy studies resulting from approved Investigational New Drugs (including vaccines).
The law requires (from Wiki):
- Federally and privately funded clinical trials;
- The purpose of each experimental drug;
- Subject eligibility criteria to participate in the clinical trial;
- The location of clinical trial sites being used for a study; and
- A point of contact for patients interested in enrolling in the trial.
- The National Library of Medicine in the National Institutes of Health to host the public website/database
(BTW, one of my former clients held the federal contract to support ClinicalTrials.gov and Pubmed. I have spent time in the back rooms of the NLM and do know a fair amount about these things….)
The searchable ClinicalTrials.gov website was made available to the public via the internet on February 29, 2000.
ClinicalTrials.gov makes searching for human clinical trials easy. For instance, a quick search reveals that there are over 50 clinical trials for mRNA vaccines in progress and over 200 registered.
With animals, there is no such database. mRNA vaccines in the “animal health” or veterinary markets are difficult to track until the company or the USDA is ready to release information on that product’s development or release. The USDA and/or the NIH have no mechanism for tracking potential new vaccines, drugs or biologics for the animal market.
Therefore, one must rely on press releases, the occasional peer reviewed paper, conference notes, USDA grant and contract notifications, university websites and company profiles for discovery of such new products. Not adequate, in my opinion, and most definitely not transparent. By federal law, the public should have open access to the results of this type of federally funded research.
In today’s substack, the state of mRNA “vaccines” for animal “health” is discussed. Citing public sources, I will review what is known and not known about commercial liaisons and partnerships, the corporations involved, ongoing research and products in various states of development.
Bayer Partners with BioNTech to Develop mRNA Vaccines, Drugs for Animal Health
Genetic Engineering and Biotechnology News. May 10, 2016
Bayer will partner with BioNTech to develop novel, first-in-class mRNA vaccines and therapeutics for animal health indications, the companies said today, under a collaboration whose value was not disclosed.
Bayer agreed to secure exclusive rights to BioNTech’s mRNA technology and intellectual property for development of mRNA vaccines for animal health applications…
The companies said their partnership is the first of its kind focused on developing mRNA therapeutics specifically for animal health applications.
…
Infectious disease vaccines is the focus of one of the three therapy platforms BioNTech is building through mRNA technologies; the other two are cancer immunotherapies and protein replacement. The three platforms are designed to produce pharmacologically optimized protein coding RNA for targeted in vivo delivery…
2016. This means that Bayer and BioNTech have been working on livestock and companion animal mRNA vaccines for over six years…
Logic predicts that they will soon have livestock and companion mRNA vaccine and RNA therapeutics on the market.
Bayer, BioNTech developing new mRNA vaccines
Feedstuffs.com May 16, 2016
Companies collaborate on cutting-edge technology to develop new solutions to protect companion and farm animal health.
Again, note the date…2016. This means that Bayer and BioNTech have been working on livestock and companion animal mRNA vaccines for over six years…
There are three therapy platforms that BioNTech has been building through mRNA technologies to be used in livestock and companion animals.
- Infectious disease vaccines
- Cancer immunotherapies and
- Protein replacement.
Bayer to manufacture mRNA vaccine in Germany
Bayer Website, February 1, 2021
“Following discussions with the German government it has become clear that current manufacturing capacities for vaccines need to be increased, particularly for potential variants of the SARS-CoV-2 virus.
This includes the need to expand production capacity as well as related manufacturing expertise in Germany.
We at Bayer will contribute even further by making more vaccine available to help fight the pandemic.
So, Bayer lent their mRNA manufacturing vaccine facilities for use for the making of COVID-19 mRNA vaccines. Given the above 2016 press releases, that Bayer and BioNtech were collaborating to make mRNA vaccines for the animal markets, it would make sense that these facilities were actually built for the production of veterinary vaccines.
SEQUIVITY: Custom Swine Vaccines, using RNA vaccines.
Merck Website, Accessed Jan 2023
Combat current and future swine diseases with SEQUIVITY from Merck Animal Health. A revolutionary swine vaccine platform, SEQUIVITY harnesses RNA particle technology to create customized prescription vaccines against strains of influenza A virus in swine, porcine circovirus (PCV), rotavirus and beyond. It’s supported by a sophisticated dashboard filled with comprehensive data and insights, all to help you stay on top.
Important to know. Merck is already selling mRNA vaccines for swine. For whatever reason, they are selling these products as “customized prescription vaccines against strains of influenza A virus in swine, porcine circovirus (PCV), rotavirus and beyond.” This is an interesting market segment. Merck’s reason to limit the production of mRNA vaccines in the “customized prescription” market is unclear. Production facility size and scaleability of the RNA product could be factors.
Acquisition Expands and Complements Merck Animal Health’s Strong Vaccine Portfolio
Merck Press Release, November 12, 2015 5:00 pm ET
MADISON, N.J., November 12, 2015 – Merck Animal Health (known as MSD Animal Health outside the United States and Canada) and Harrisvaccines, Inc., today announced the companies have entered into an agreement under which Merck Animal Health will acquire Harrisvaccines, a privately-held company that develops, manufactures and sells vaccines for food production and companion animals.
“As a leader in biologics, Merck Animal Health has built a robust portfolio of vaccines across all animal species,” stated Rick DeLuca, president, Merck Animal Health. “Combining Harrisvaccines’ R&D and portfolio of products with our strong capabilities and global reach will enable us to address even more devastating diseases that are impacting production animals and reinforce our commitment to the science of healthier animals.”
Harrisvaccines offers innovative technology and an important portfolio of vaccines, with a focus on production animals, an increasingly important segment as consumer demand for protein continues to grow worldwide. The company has a unique RNA Particle technology which represents a breakthrough in vaccine development. It also has a highly versatile production platform able to target a wide range of viruses and bacteria. Pathogens are collected from a farm and specific genes are sequenced and inserted into RNA particles, making safe, potent vaccines able to provide herd-specific protection.
This pioneering system is rapidly adaptable to new disease challenges and was instrumental in producing the first conditionally licensed vaccine to help control Porcine Epidemic Diarrhea Virus (PEDv), a deadly virus that has killed more than eight million piglets since suddenly emerging in the U.S. in 2013.
Read that last paragraph again. Slowly.
Sometime before 2015, the USDA issued a conditional license for a mRNA vaccine for use in pigs for Porcine Epidemic Diarrhea Virus (PEDv), information about this product can be found at drugs.com.
Basically something akin to an emergency use authorization was issued around 2014 or 2015. Just like with the mRNA COVID-19 vaccine, full licensure was not granted but the conditional license remains in place. Is this a strategy to circumvent the USDA vaccine licensing and/or authorization process?
To conclude:
Like with the BioNtech’s veterinary mRNA vaccine development, Merck’s development of an mRNA vaccine product started years ago. For Merck, it may have begun in earnest in 2015 with the acquisition of Harris Vaccine.
Some ongoing research:
NOVEL MRNA VACCINE TECHNOLOGY FOR PREVENTION OF BOVINE RESPIRATORY SYNCYTIAL VIRUS
IOWA STATE UNIVERSITY (grant summary page)
Non Technical Summary
Bovine respiratory syncytial virus (RSV) is a significant viral pathogens of young cows that is a key component of the respiratory disease complex and often leads to secondary bacterial pneumonia. Prefusion F has recently shown to be highly efficacious in barrier housed RSV challenged cows. However, the difficulty in generating prefusion F along with the cost of its production are a hurdle for adoption to the farm. RSV immunity also tends to wane quickly and given the complications of field or pen raised cattle and their stressors and other circulating diseases, and a protein vaccine may not prove highly efficacious in the real world. Here, we will test a novel mRNA vaccine system we have developed that substantially lowers the price point for production animals and may lead to more thermal stable transcripts compatible with vaccinating on the farm. The use of an alternative delivery system rather than lipid nanoparticles will also lower the vaccine costs. We expect to demonstrate efficacy of the vaccine platform using mice at first as proof of principle before switching to a full cow vaccination and challenge system in year 2. Our overall goal is to test a novel mRNA system for inducing immunological protection from bovine RSV infection. We hypothesize that a prefusion F mRNA delivered continuously by vaccine implant will lead to prolonged and robust cellular and antibody immunity. Here, we will optimize our vaccine further and then test for potential correlates of protection to examine for in eventually challenged cows.
Research into mRNA vaccine livestock vaccines in New Zealand and Australia continues with governmental fast-track approval.
NSW fast tracks mRNA FMD and Lumpy Skin Disease vaccines (in cattle)
The NSW Government has taken another step towards fast tracking the world first mRNA vaccines for Foot and Mouth Disease (FMD) and Lumpy Skin Disease, inking a deal with US biotechnology company Tiba Biotech
A Foot and Mouth Disease mRNA Vaccine Deal Has Been Signed Between the NSW Government and US Company Tiba Biotech
Finally, inquiring minds want to know… what is Pfizer up to?
Pfizer animal health goes by the name Zoetis.
Zoetis clearly does not make its animal vaccine developmental stages known to the public. Internet searches do not reveal much inside the workings of Zoetis, in terms of mRNA vaccines. However, we can safely assume that development of mRNA vaccines and therapeutics for “animal health” are underway – so stay tuned.
Finally, there are mRNA vaccines for COVID-19 for wildlife that have been developed and authorized for distribution by the USDA.
Black-footed ferret COVID-19 vaccination seems to be working
The Wildlife Society, Feb 18, 2021
After finding similar species can be infected, researchers quickly began to increase safety protocols [distancing, not vaccination] at zoos and the U.S. Fish and Wildlife Service’s National Black-footed Ferret Conservation Center in Colorado, the main source of the captive-breeding and release program for the federally endangered species.
“They have done a magnificent job in keeping those animals safe,” said Tonie Rocke, a research scientist with the USGS National Wildlife Health Center who works with ferrets.
But U.S. Geological Survey researchers who also study black-footed ferrets had learned about recent studies in mice and hamsters, demonstrating safety and efficacy of vaccination against COVID-19 using purified viral protein. They decided to try something similar on a handful of ferrets this past May and June.
The vaccine used in ferrets is different — it’s a simplified version of the Moderna or Pfizer vaccinations now being used for humans — and it’s based on a similar protein, said Rocke.
Under the authority of the USFWS, the scientists could test the solution on a handful of ferrets in a process that is much quicker than the extensive approvals needed for commercial vaccination for humans like the Pfizer or Moderna inoculations.
The isolated ferrets that had received this trial vaccination produced antibodies against the coronavirus.
Unfortunately, I could find no updates to this program and whether it was expanded into other wildlife populations.
Again, something akin to an emergency use authorization was issued for this experimental vaccine. Just like with the mRNA COVID-19 vaccine and the RNA porcine vaccine above, full licensure was not granted but it appears that the conditional license remains in place. I raise the question again, is this a USDA and/or corporate strategy to circumvent the USDA vaccine licensing and/or authorization process?
The issue being of course, that there is no mechanism for “right to know” of animal health vaccine development.
Conclusion: There were news stories in 2020 that mRNA vaccine(s) were being developed for COVID/SARS-CoV-2 for administration to livestock and companion animals. However, the lack of updates suggest that these plans may have been scrapped with the new, less virulent variants.
Shared from https://rwmalonemd.substack.com/p/mrna-vaccines-in-livestock-and-companion